Landus Mumbere Expedito
|Subscribers
Latest videos
Designing a brochure is just easy to use templates
On the Start page that appears when you open Publisher, click Brochure (You can get to the Start page anytime by clicking File -New). Search for Brochures.
or design from scratch. In This video, we shall design from scratch.
Below is the Question
Use Desktop publishing software to prepare a brochure for JK Computer Center with the following details.
Company Name: JK Computer Center
Motto: Computer skills for better standards.
Location: Seventh Street, Jinja Road
Address: P.O Box 38, Kampala
Telephone Contact: 0775610762, 0701517817, 0758767895
E-mail Address: jkcomputercenter@gmail.com
Website: https://www.jkcomputer.net
Introduction: JK Computer Center is a home of computer services and accessories. We have customer centered service providers. Please come and enjoy our excellent services.
Services offered: Internet services, Printing, Photocopying, Scanning, Binding, Mobile Money, Computer and phone repair, Computer and phone accessories, Airtime selling, Telecommunications services, Report writing, Typing and Computer lessons.
(a) Divide each page into three panels
(b) Enter the given details in suitable panels
(c) Use appropriate images, fonts and background in the brochure.
(d) Add your name and personal number as footer
(e) Save your brochure as your name and personal number.
(f) Print your work.
More Video Lessons on Desktop Publishing By Kakuru Benard
Lesson 1: https://youtu.be/QRzLXw7gx_o
Lesson 2: https://youtu.be/CbLR6mi9hOk
Lesson 3: https://youtu.be/KGXxogpB6B0
Lesson 4: https://youtu.be/dzJP70T1poc
Lesson 5: https://youtu.be/xNMz5tfEkW0
Lesson 6: https://youtu.be/F85pzjaQYgI
Lesson 7: https://youtu.be/12WPjN92Sns
Lesson 8: https://youtu.be/L_EZbCC05tc
Wakisha 2023: https://youtu.be/U5-oYEIUn5o
UNEB 2023 Paper 2: https://youtu.be/jAgtwfS6OC8
UNEB 2023 Paper 3: https://youtu.be/E2bwpS-Gwgw
UNEB 2023 Paper 1: https://youtu.be/GWRXjetAjpg
Create a database for Jett Car Hire Ltd saved as your name and personal number and carry out the following tasks.
Instructions:
(a). Design a table saved as drivers to hold the above data.
(b). Design a tabular form having a sky-blue background colour, footer of your name you will use to populate the table. Save the form as Data Entry.
(c). Design three queries that will return workers who:
(i). have no remarks against their records. Save the query as Not Appraised.
(ii). drive Car_Type that is not a Wish. Save the query as Not Wish.
(iii). celebrate birth day in the month of January. Save the query as Born Jan.
(d). Create a report to return drivers who drive a Premio Car_Type. Save the report as Premio.
(e) Create a report having all the records on one sheet. Save the report as All.
The report should have the following details:
(i). A good red line boarder.
(ii). Group and sort your records in order of car_type
(iii). Add a title: MOTO VEHICLES INFORMATION REPORT size 24
(f) Add an image Motor.png on your report to serve as a logo
(g) Print your queries and report only.
More Video Lessons On Database Access By Kakuru Benard
Lesson 1: https://youtu.be/dJaCGMqGJvc
Lesson 2: https://youtu.be/Y3W9DVcgEPU
Lesson 3: https://youtu.be/qNnvdX1ibRk
Lesson 4: https://youtu.be/41UWvApgg58
Lesson 5: https://youtu.be/REQESvACSSs
Lesson 6: https://youtu.be/RoXjFYqwcSo
Lesson 7: https://youtu.be/NIjSEl5Kopw
Lesson 8: https://youtu.be/FUdtRE4fKf8
Lesson 9: https://youtu.be/n5PDGCFKyLM
Wakisha 2023: https://youtu.be/U5-oYEIUn5o
UNEB 2023 Paper 2: https://youtu.be/jAgtwfS6OC8
UNEB 2023 Paper 3: https://youtu.be/E2bwpS-Gwgw
UNEB 2023 Paper 1: https://youtu.be/GWRXjetAjpg
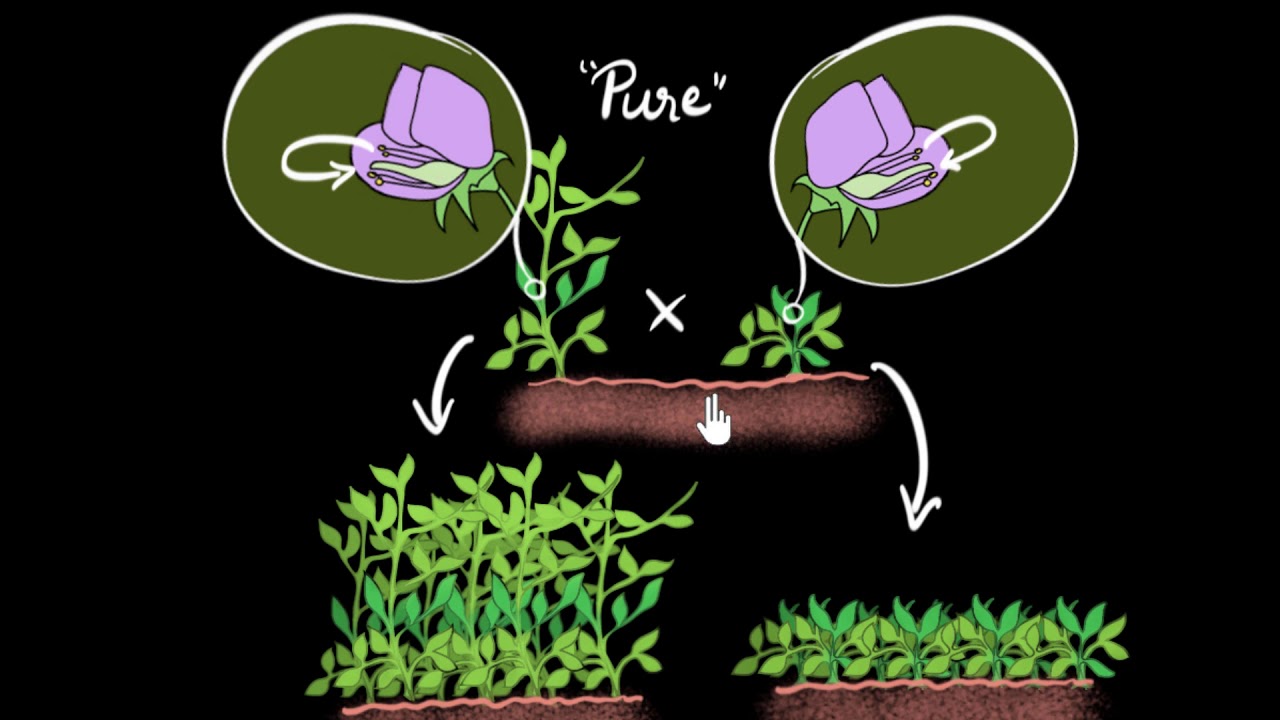
Let's explore the exciting pea plant experiment by Mendel
More free lessons & practice -https://www.khanacademy.org/sc....ience/in-in-class9th
Khan Academy is a nonprofit organization with the mission of providing free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Created by Mahesh Shenoy
Let's learn the 5 major plant hormones and how we can remember their functions.
More free lessons & practice -https://www.khanacademy.org/sc....ience/class-10-biolo
Khan Academy is a nonprofit organisation with the mission of providing free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Created by Mahesh Shenoy
Let's explore the law of independent assortment
More free lessons & practice -https://www.khanacademy.org/sc....ience/class-10-biolo
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Created by Mahesh Shenoy
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/sc....ience/physics/one-di
An overview video of what physics is about as we delve deeper in future videos!
Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physical situations. To get the most out of physics, you'll need a solid understanding of algebra and a basic understanding of trigonometry.
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #youcanlearnanything
subscribe to Khan Academy’s Physics channel: https://www.youtube.com/channe....l/UC0oGarQW2lE5PxhGo
Subscribe to Khan Academy: https://www.youtube.com/subscr....iption_center?add_us
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/sc....ience/cosmology-and-
Gravity, Weak, Electromagnetic and Strong. Created by Sal Khan.
Watch the next lesson: https://www.khanacademy.org/science/cosmology-and-astronomy/universe-scale-topic/big-bang-expansion-topic/v/big-bang-introduction?utm_source=YT&utm_medium=Desc&utm_campaign=cosmologystronomy
Missed the previous lesson? https://www.khanacademy.org/science/cosmology-and-astronomy/universe-scale-topic/light-fundamental-forces/v/introduction-to-light?utm_source=YT&utm_medium=Desc&utm_campaign=cosmologystronomy
Cosmology & Astronomy on Khan Academy: The Earth is huge, but it is tiny compared to the Sun (which is super huge). But the Sun is tiny compared to the solar system which is tiny compared to the distance to the next star. Oh, did we mention that there are over 100 billion stars in our galaxy (which is about 100,000 light years in diameter) which is one of hundreds of billions of galaxies in just the observable universe (which might be infinite for all we know). Don't feel small. We find it liberating. Your everyday human stresses are nothing compared to this enormity that we are a part of. Enjoy the fact that we get to be part of this vastness!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #youcanlearnanything
subscribe to Khan Academy’s Cosmology & Astronomy channel: https://www.youtube.com/channe....l/UChNPnEkW8LYZ5Rwi8
Subscribe to Khan Academy: https://www.youtube.com/subscr....iption_center?add_us
In case you missed it, this is a Physics video! Also, are spherical cows the key to understanding the universe, or maybe not? Not sure. Anyway, learn why modelling and approximations in Physics can be immensely useful!
To get you fully ready for your exam and help you fall in love with Physics, find the complete bank of exercises and videos for Class 11 Physics here - https://www.khanacademy.org/sc....ience/in-in-class11t
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Vibhor Pandey
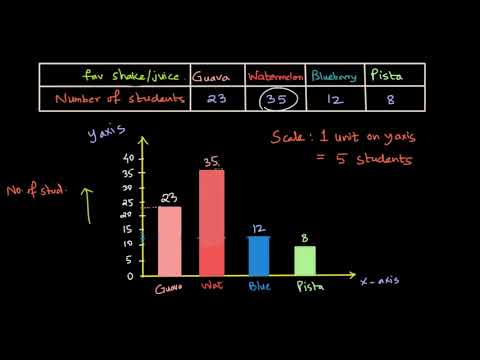
In this video we discuss how we can chose the scale for drawing the bar graph and also why choosing the scale is important.
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Devahsish Phadnis
In this video we discuss what is an altitude of a triangle and how we can draw/identitfy different altitudes of the triangle
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Devashish Phadnis
We look at the exterior angle property of the triangle and a quick problem on how to apply it.
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Devashish Phadnis
In this video we discuss the properties of the equilateral and isosceles triangles.
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Devashish Phadnis
Let's figure out the charge on a capacitance in a mixed circuit.
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Vibhor Pandey
Let's solve a question on Bernoulli's principle!
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Vibhor Pandey
Let's figure out the value of phase constant under certain conditions!
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Vibhor Pandey
Let's solve a question on equivalent thermal conductivity
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Vibhor Pandey
Let's solve a question on power dissipated in two bulbs connected in series
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy.
Subscribe to our YouTube channel - https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by
In this video, we talk about population density and how difficult it can get to estimate sometimes. We find out how to count tiger populations and that population density isn't always about the number of individuals in the area.
Timestamps:
00:00 - What is population density?
01:46 - Purpose of population density
02:49 - How to count tigers?
04:58 - Biomass density as an alternative
07:27 - Summary
Practice this concept: https://www.khanacademy.org/sc....ience/in-in-class-12
Master the concept of Organisms and their Environments through practice exercises and videos: https://www.khanacademy.org/sc....ience/in-in-class-12
Check out more videos and exercises on Organisms and Populations: https://www.khanacademy.org/sc....ience/in-in-class-12
To get you fully ready for your exam and help you fall in love with biology, find the complete bank of exercises and videos for Class 12 Biology here: https://www.khanacademy.org/sc....ience/in-in-class-12
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy!
Subscribe to our YouTube channel: https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Sulagna Das
In this video, we explore how today's population can help us predict how it will be like in the future. And, talk about the showrunner of this prediction: the age pyramids.
Timestamps:
00:00 - What is an age pyramid?
01:52 - Expanding age pyramid
05:44 - Declining age pyramid
07:48 - Stable age pyramid
09:32 - India's age pyramid
Practice this concept: https://www.khanacademy.org/sc....ience/in-in-class-12
Master the concept of Organisms and their Environments through practice exercises and videos: https://www.khanacademy.org/sc....ience/in-in-class-12
Check out more videos and exercises on Organisms and Populations: https://www.khanacademy.org/sc....ience/in-in-class-12
To get you fully ready for your exam and help you fall in love with biology, find the complete bank of exercises and videos for Class 12 Biology here: https://www.khanacademy.org/sc....ience/in-in-class-12
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy!
Subscribe to our YouTube channel: https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Sulagna Das
In this video, we explore the concept of adaptations and how different types of adaptations have allowed organisms to survive in the most bizarre environments without a glitch.
Timestamps:
01:42 - Structural adaptations
07:20 - Physiological adaptations
09:15 - How Tibetans can live 13,000 feet in the mountains?
12:02 - Behavioural adaptations
14:15 - Definition of adaptation
Practice this concept: https://www.khanacademy.org/sc....ience/in-in-class-12
Master the concept of Organisms and their Environments through practice exercises and videos: https://www.khanacademy.org/sc....ience/in-in-class-12
Check out more videos and exercises on Organisms and Populations: https://www.khanacademy.org/sc....ience/in-in-class-12
To get you fully ready for your exam and help you fall in love with biology, find the complete bank of exercises and videos for Class 12 Biology here: https://www.khanacademy.org/sc....ience/in-in-class-12
Khan Academy is a free learning platform for Class 1-12 students with videos, exercises, and tests for maths, science, and more subjects. Our content is aligned to CBSE syllabus and available in Hindi, English, and many more regional languages.
Experience the joy of easy, seamless, accessible learning anywhere, anytime with Khan Academy!
Subscribe to our YouTube channel: https://www.youtube.com/c/khanacademy
As a 501(c)(3) nonprofit organization, we would love your help!
Donate here: https://www.khanacademy.org/donate?utm_source=youtube&utm_medium=desc
Created by Sulagna Das