Organic chemistry lesson 12
Classification of aminesboiling and melting points of aminesolubility of aminesbasicity of aminespreparation of amines from alkyl halides, cyanides, nitroalkanesHofmann's degredation
## Amines: Comprehensive Notes
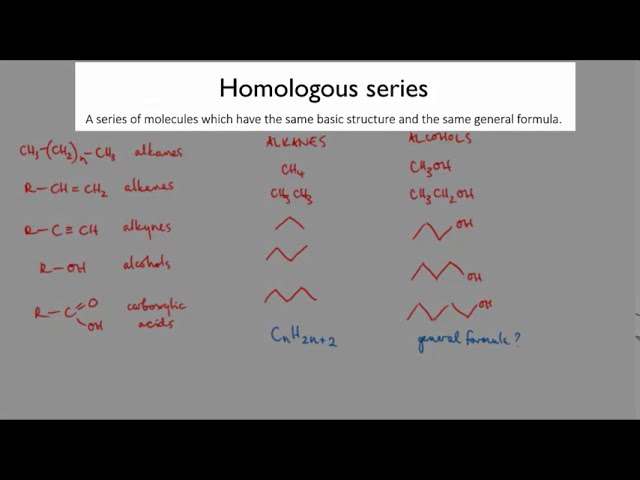
**1. Classification:**
Amines are organic compounds derived from ammonia (NH₃) by replacing one or more hydrogen atoms with alkyl or aryl groups. They are classified into four primary types based on the number of alkyl/aryl groups attached to the nitrogen atom:
* **Primary (1°):** One alkyl/aryl group attached to the nitrogen atom (e.g., CH₃NH₂ - Methylamine)
* **Secondary (2°):** Two alkyl/aryl groups attached to the nitrogen atom (e.g., (CH₃)₂NH - Dimethylamine)
* **Tertiary (3°):** Three alkyl/aryl groups attached to the nitrogen atom (e.g., (CH₃)₃N - Trimethylamine)
* **Quaternary (4°):** All four positions around the nitrogen atom are occupied by alkyl/aryl groups, resulting in a positively charged cation (e.g., [(CH₃)₄N⁺]Cl⁻ - Tetramethylammonium chloride)
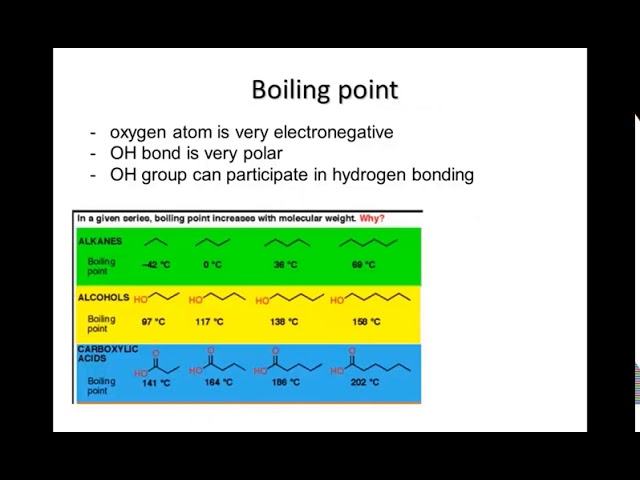
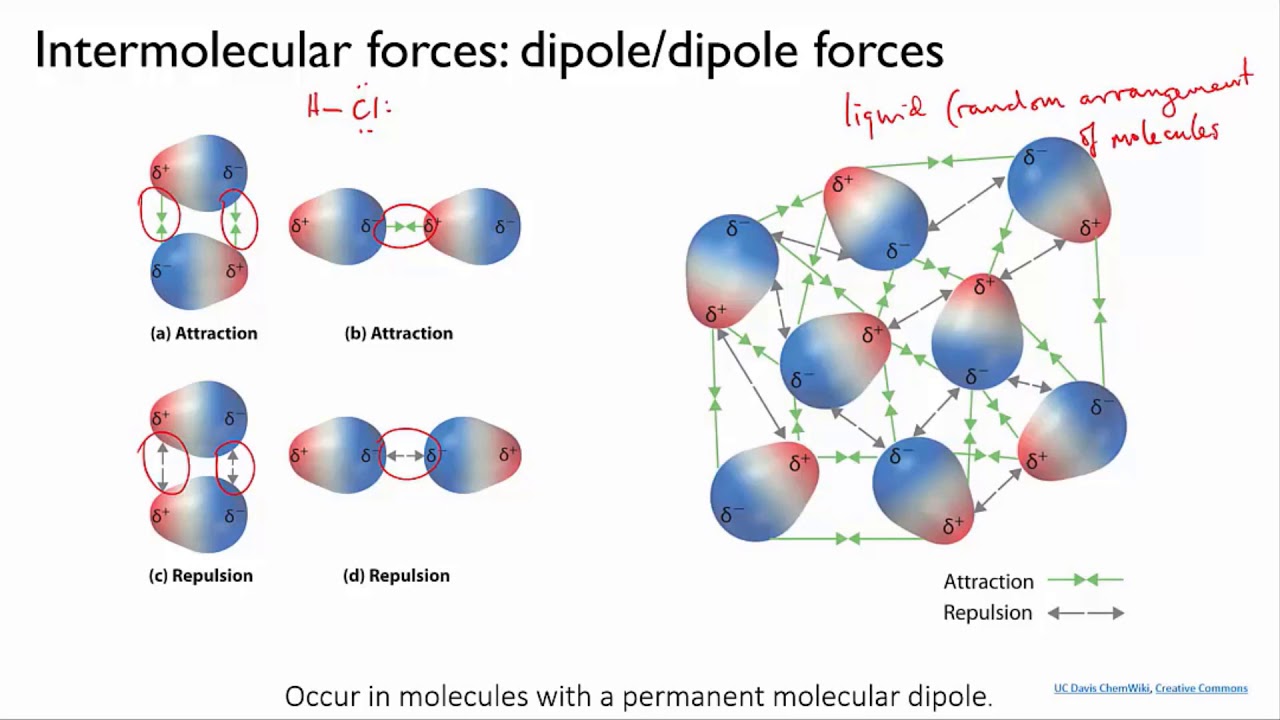
**2. Physical Properties:**
* **Boiling and Melting Points:**
* Generally increase with increasing chain length of the alkyl/aryl groups due to stronger London dispersion forces.
* Branching in the chain can decrease boiling and melting points due to a decrease in surface area and weaker intermolecular interactions.
* Amines generally have lower boiling and melting points compared to similarly sized alcohols due to the absence of hydrogen bonding in amines.
* **Solubility:**
* Lower amines (primary and secondary) exhibit good water solubility due to the ability to form hydrogen bonds with water molecules.
* Solubility in water decreases with increasing size and branching of the alkyl/aryl groups due to the dominance of hydrophobic interactions over hydrogen bonding.
* Tertiary and quaternary amines with no N-H bonds are typically less soluble in water but may show solubility in organic solvents.
**3. Basicity:**
Amines exhibit basic character due to the presence of a lone pair of electrons on the nitrogen atom. This lone pair can accept a proton from acids, forming a positively charged ammonium ion. The basicity of amines follows the trend:
**Tertiary > Secondary > Primary > Ammonia**
Several factors influence the basicity of amines:
* **Inductive effect:** Electron-donating groups (e.g., alkyl groups) attached to the nitrogen atom increase basicity by pushing electron density towards the nitrogen, making it more willing to accept a proton.
* **Steric hindrance:** Bulky groups around the nitrogen atom hinder the approach of a proton, decreasing basicity.
**4. Preparation:**
Amines can be synthesized through various methods, some of the most common being:
* **Alkylation of ammonia or amines:** Reaction of ammonia or amines with alkyl halides under suitable conditions (e.g., heating with KOH).
* **Reduction of nitriles:** Conversion of nitriles (R-CN) to primary amines (R-CH₂NH₂) using reducing agents like LiAlH₄ or catalytic hydrogenation.
* **Reduction of nitroalkanes:** Conversion of nitroalkanes (R-NO₂) to primary, secondary, or tertiary amines depending on the reaction conditions and reducing agent used.
* **Gabriel synthesis:** Synthesis of primary amines from phthalimide using strong bases and alkyl halides.
**5. Hofmann Degradation:**
This reaction allows the conversion of a primary amide to a primary amine with one fewer carbon atom. The process involves treating the amide with bromine (Br₂) and sodium hydroxide (NaOH), leading to rearrangement and cleavage of the carbon-nitrogen bond.
**6. Applications of Amines:**
Amines have diverse applications in various fields, including:
* **Pharmaceuticals:** Many drugs, such as antidepressants, decongestants, and antihistamines, contain amine groups.
* **Dyes and pigments:** Amines are used in the production of various dyes and pigments used in textiles, paints, and plastics.
* **Polymers and resins:** Amines are essential components in the synthesis of various polymers like nylon and resins used in adhesives and coatings.
* **Agrochemicals:** Some herbicides and insecticides contain amine functionalities.
* **Surfactants:** Quaternary ammonium salts are used as cationic surfactants in detergents and fabric softeners.
**7. Safety Considerations:**
Amines can exhibit various toxicities depending on their structure and properties. It is crucial to handle them with appropriate safety precautions, including wearing gloves, eye protection, and working in well-ventilated areas. Some amines may be flammable or corrosive, and proper handling procedures should be followed.