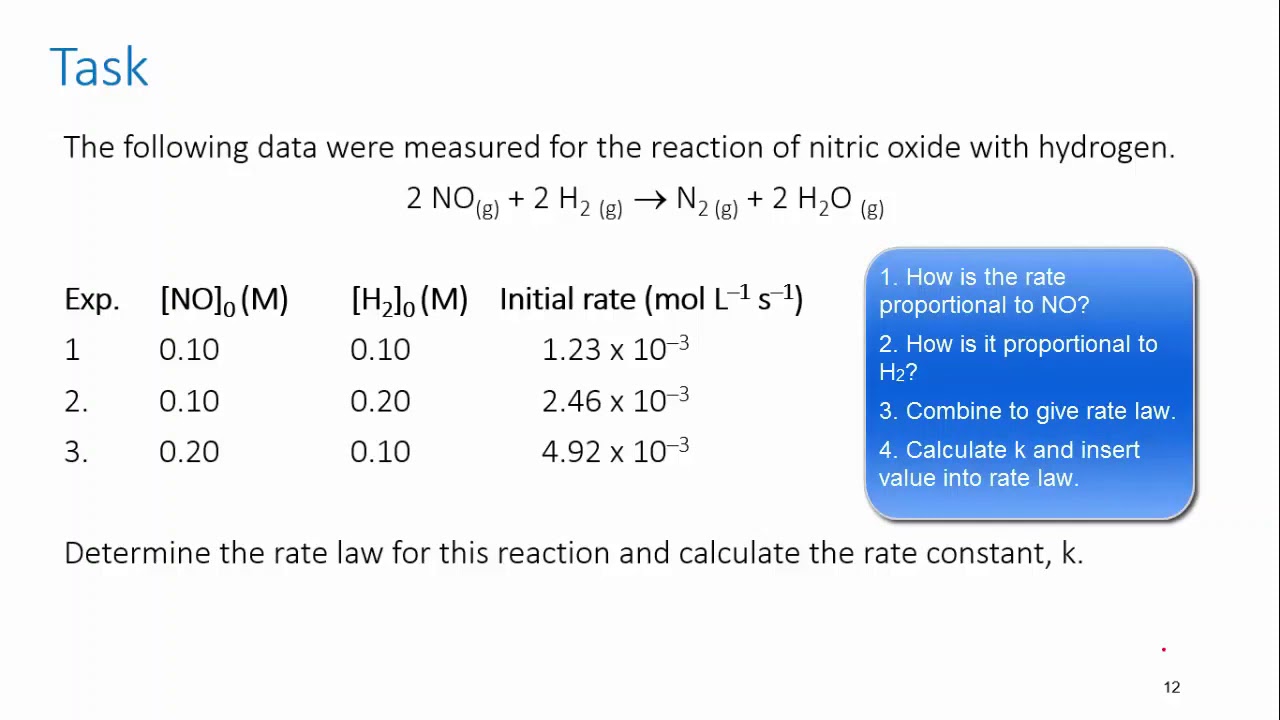
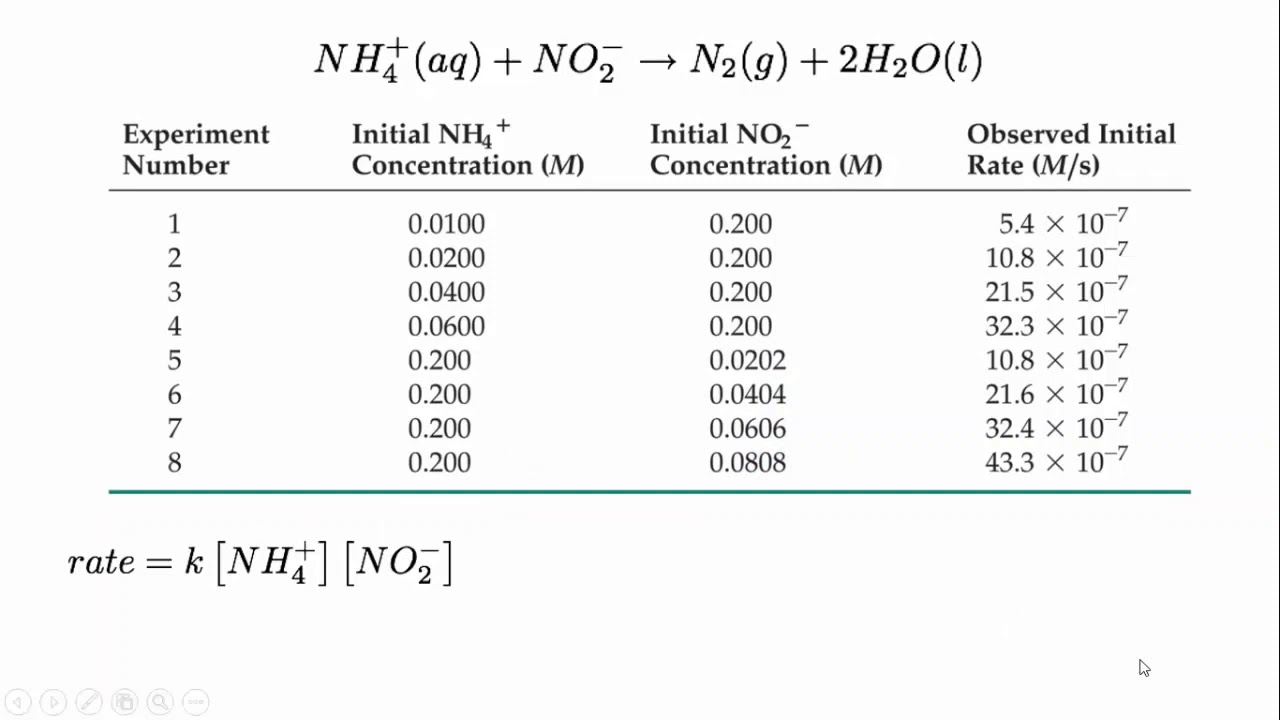
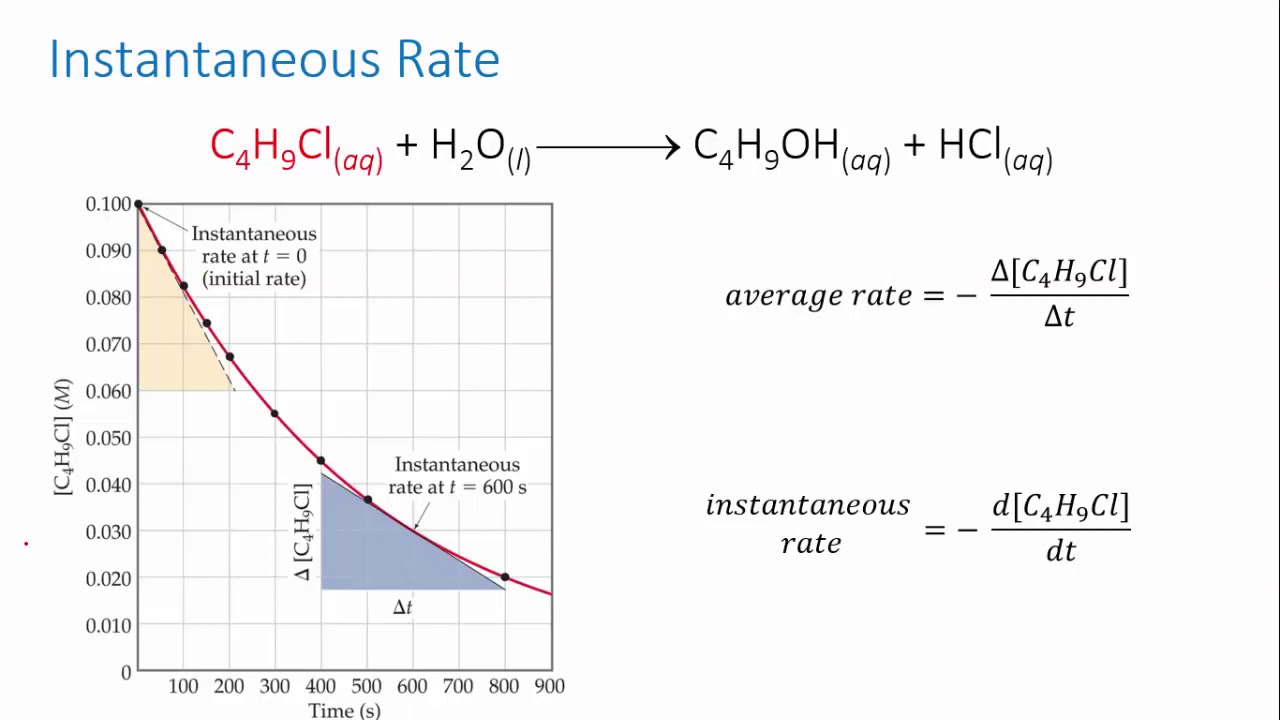
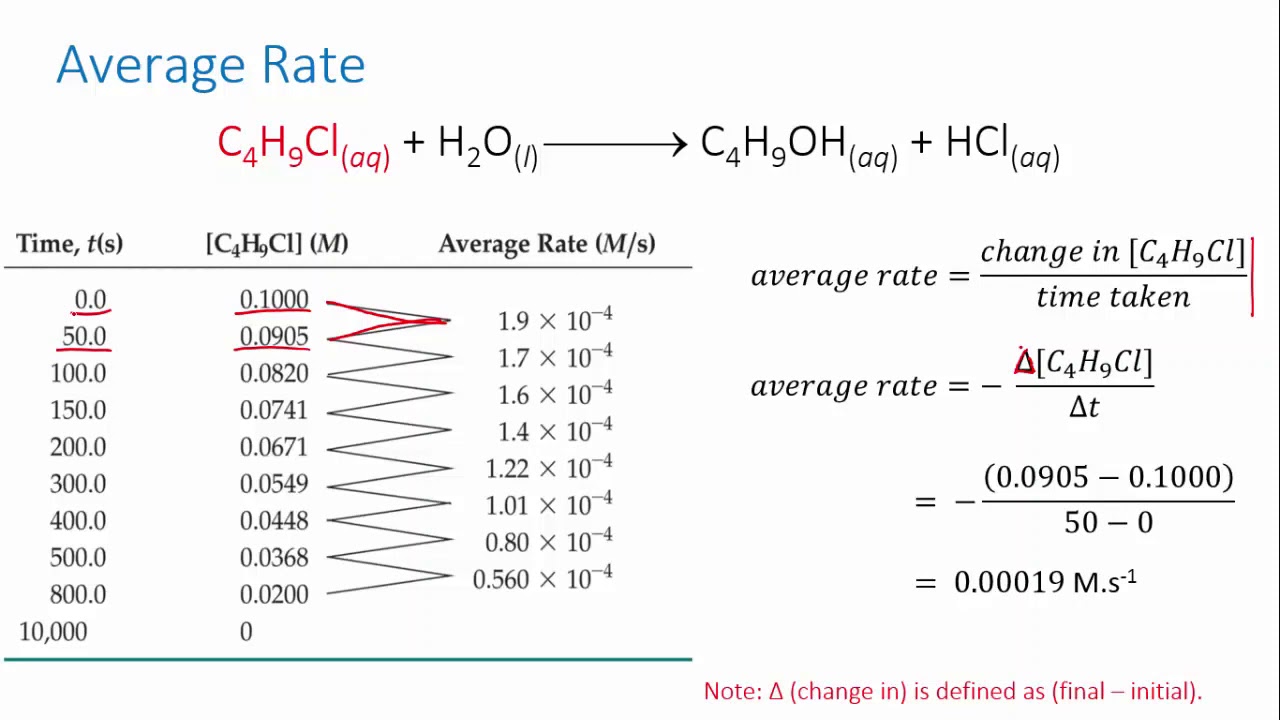
CHEM REACTION RATES & REVERSIBLE REACTION
Reaction Rates:
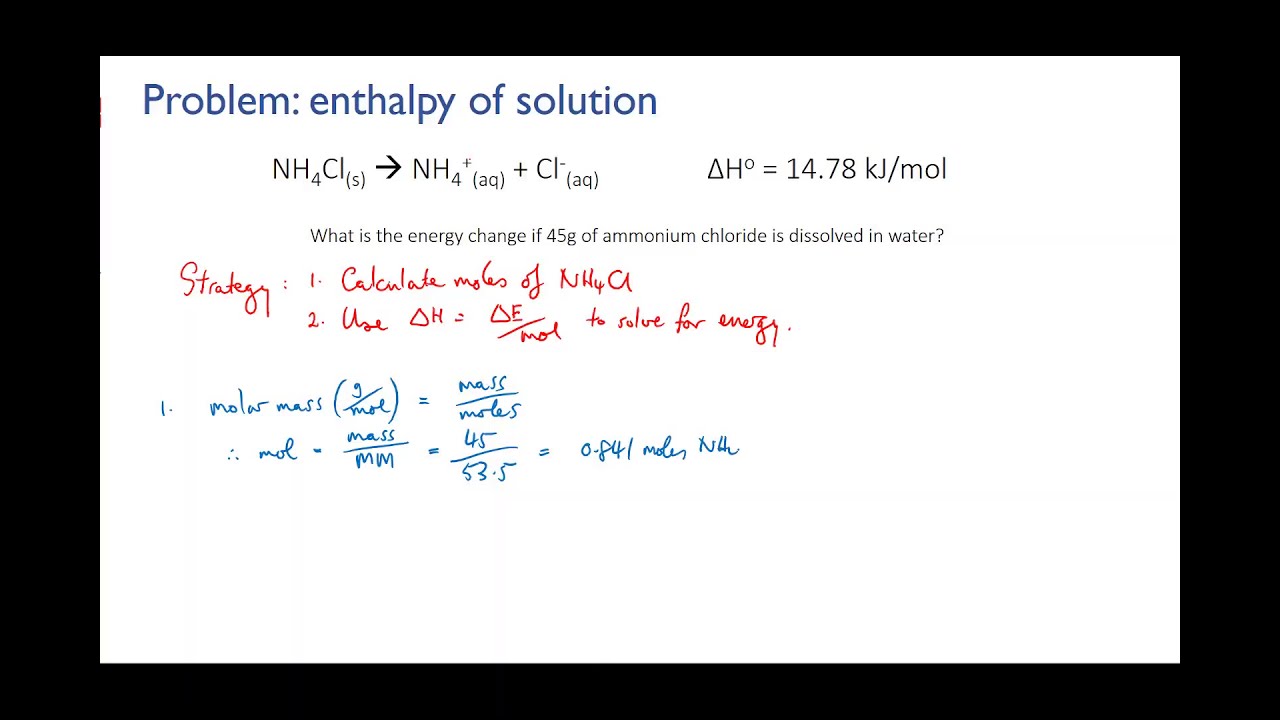
Reaction rate is a measure of how fast a chemical reaction takes place. It is often described as the change in concentration of a reactant or product per unit of time. The rate of a chemical reaction is influenced by several factors, including temperature, concentration of reactants, surface area, and the presence of catalysts.
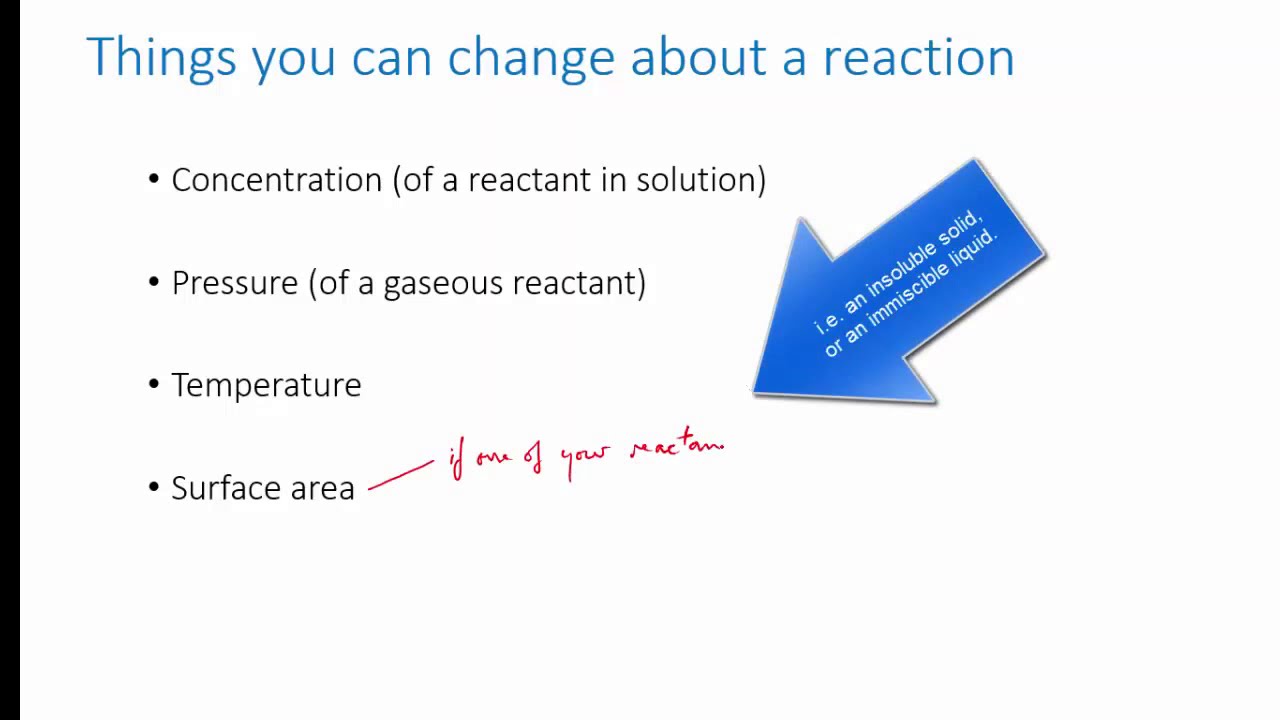
Temperature: Increasing the temperature generally increases the reaction rate. This is because higher temperatures provide more energy to the reactant particles, causing them to move faster and collide with greater force. This leads to a higher frequency of effective collisions, resulting in a faster reaction rate.
Concentration: Increasing the concentration of reactants typically increases the reaction rate. This is because a higher concentration means there are more reactant particles present, leading to a higher frequency of collisions and a greater likelihood of effective collisions.
Surface Area: Increasing the surface area of solid reactants can increase the reaction rate. This is because greater surface area provides more opportunities for reactant particles to come into contact with each other, increasing the frequency of collisions.
Catalysts: Catalysts are substances that increase the reaction rate by providing an alternative pathway for the reaction with lower activation energy. They do not get consumed in the reaction and can be reused. Catalysts work by reducing the energy barrier required for the reaction to occur, allowing more reactant particles to overcome this barrier and participate in the reaction.
Reversible Reactions:
Reversible reactions are chemical reactions that can proceed in both the forward and reverse directions. This means that reactants can form products, and products can also react to form the original reactants. Reversible reactions are often represented by a double-headed arrow, indicating that the reaction can occur in both directions.
An example of a reversible reaction is the formation of water from hydrogen and oxygen:
2H2 + O2 ⇌ 2H2O
In this reaction, hydrogen and oxygen can react to form water, and water can also decompose to form hydrogen and oxygen. The double-headed arrow represents the equilibrium between the forward and reverse reactions.
In a reversible reaction, the reaction rate of the forward and reverse reactions can be different. The extent to which a reversible reaction proceeds in the forward direction depends on the relative concentrations of reactants and products. At equilibrium, the reaction rates of the forward and reverse reactions become equal, and there is no net change in the concentrations of reactants and products.
Factors such as temperature, pressure, and concentration can affect the position of equilibrium in a reversible reaction. These factors can shift the equilibrium towards the formation of more products or reactants.