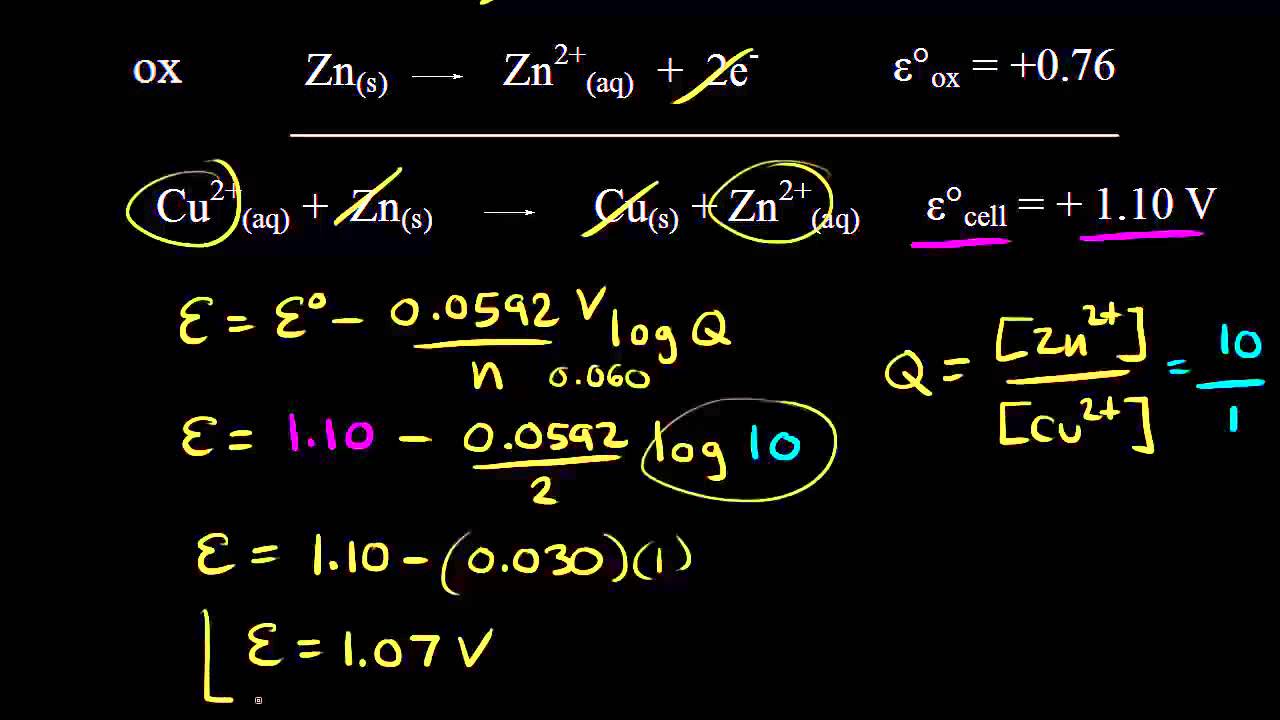ELECTROCHEMISTRY ( Principles of electrolysis) (Selective discharge of ions)
## Principles of Electrolysis: Selective Discharge of Ions
Electrolysis involves the use of electrical energy to drive a non-spontaneous chemical reaction. During this process, an electric current flows through an **electrolyte** (a solution containing ions) and causes the ions within to migrate towards the electrodes. However, not all ions are created equal!
The concept of **selective discharge of ions** is crucial in understanding how electrolysis works. It refers to the phenomenon where **only certain ions are discharged** (gain or lose electrons) at the electrodes, while others remain in the solution un-reacted.
Here are the key factors influencing which ions get discharged:
**1. Reactivity Series:**
- Metals higher in the reactivity series are **more easily oxidized** (lose electrons) and therefore **less likely to be discharged** at the cathode. (e.g., Sodium, Potassium)
- Conversely, metals **lower in the series** are **more difficult to oxidize** and are **more likely to be discharged** at the cathode. (e.g., Copper, Silver)
**2. Ion Concentration:**
- Ions present in **higher concentrations** are **more likely to be discharged** than those present in lower concentrations. This is simply due to the increased number of collisions between the ions and the electrode.
**3. Electrode Material:**
- The material of the electrode can also play a selective role. Some electrodes have a **higher affinity for specific ions** compared to others.
**Overall process:**
1. **Cations (positive ions)** are attracted to the **cathode (negative electrode)**, where they **gain electrons and are reduced**.
2. **Anions (negative ions)** are attracted to the **anode (positive electrode)**, where they **lose electrons and are oxidized**.
The specific reactions at the electrodes depend on the specific electrolyte and electrode materials used. However, the **selective discharge of ions** remains the fundamental principle governing which ions react and how.











![Principles of Software Development Lifecycle [Dev Concepts #25]](https://i.ytimg.com/vi/dhf4-LVuN2E/maxresdefault.jpg)




![D3.2 Principles of Inheritance [IB Biology SL/HL]](https://i.ytimg.com/vi/YRCCuPvOA0s/maxresdefault.jpg)