Form 4 chemistry - Reaction Rates Lesson 1
Reaction rates and reversible reactions are important concepts in chemistry that are closely related.
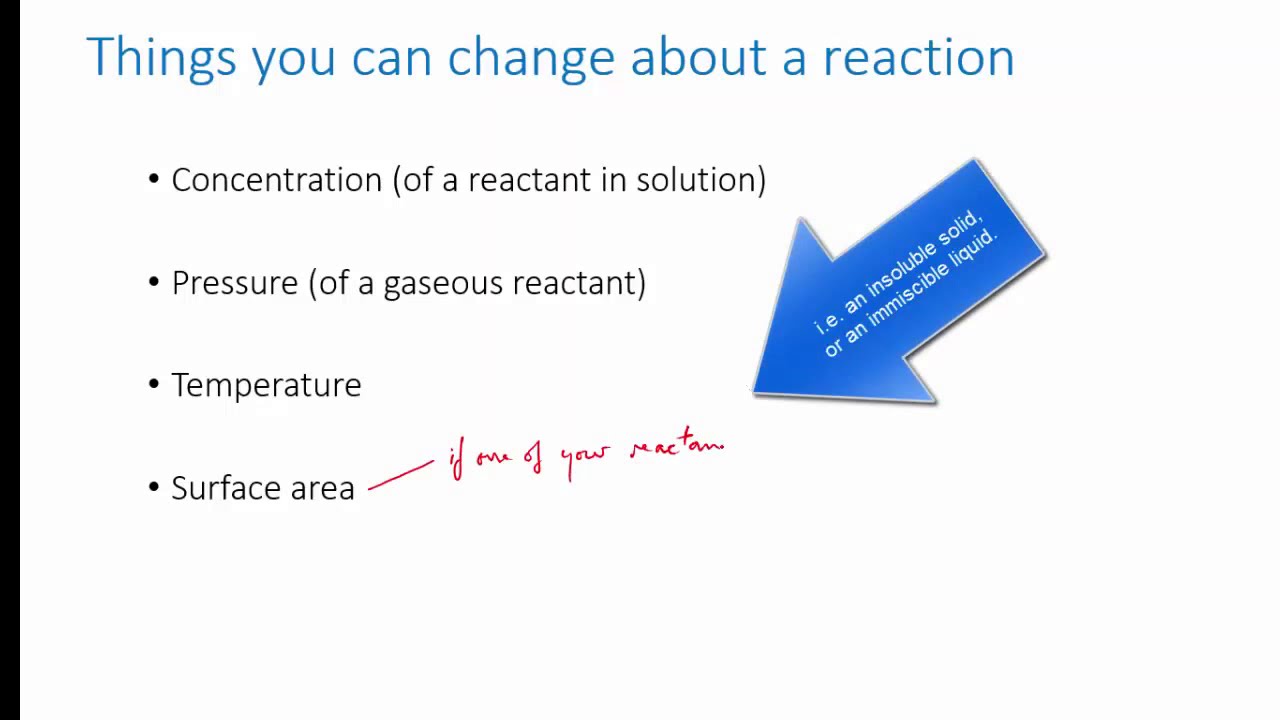
Reaction rates refer to the speed at which a chemical reaction takes place. It measures how quickly reactants are consumed or how quickly products are formed during a reaction. Reaction rates can be influenced by various factors such as temperature, concentration, pressure, and the presence of catalysts.
On the other hand, reversible reactions are reactions that can proceed in both the forward and reverse directions. This means that reactants can form products, and products can also react to form the original reactants. Reversible reactions are denoted by a double-headed arrow (⇌) to indicate their bidirectional nature.
In reversible reactions, the forward and reverse reactions occur simultaneously, but the reaction rates may be different. The rate of the forward reaction is determined by the concentrations of the reactants, while the rate of the reverse reaction is determined by the concentrations of the products. At equilibrium, the rates of the forward and reverse reactions become equal, and the concentrations of reactants and products remain constant.
The reaction rate of a reversible reaction can be influenced by factors such as temperature, pressure, and concentration. Changing these factors can alter the position of equilibrium, which refers to the relative amounts of reactants and products in a reversible reaction system. For instance, increasing the temperature usually shifts the equilibrium towards the endothermic direction, while increasing the pressure may favor the formation of products with fewer moles.
Understanding the relationship between reaction rates and reversible reactions is important for studying chemical kinetics and thermodynamics. It allows scientists to predict and control the rates and outcomes of chemical reactions. Knowledge of reaction rates and reversible reactions is particularly useful in industrial processes, where optimizing reaction conditions can improve efficiency and yield.