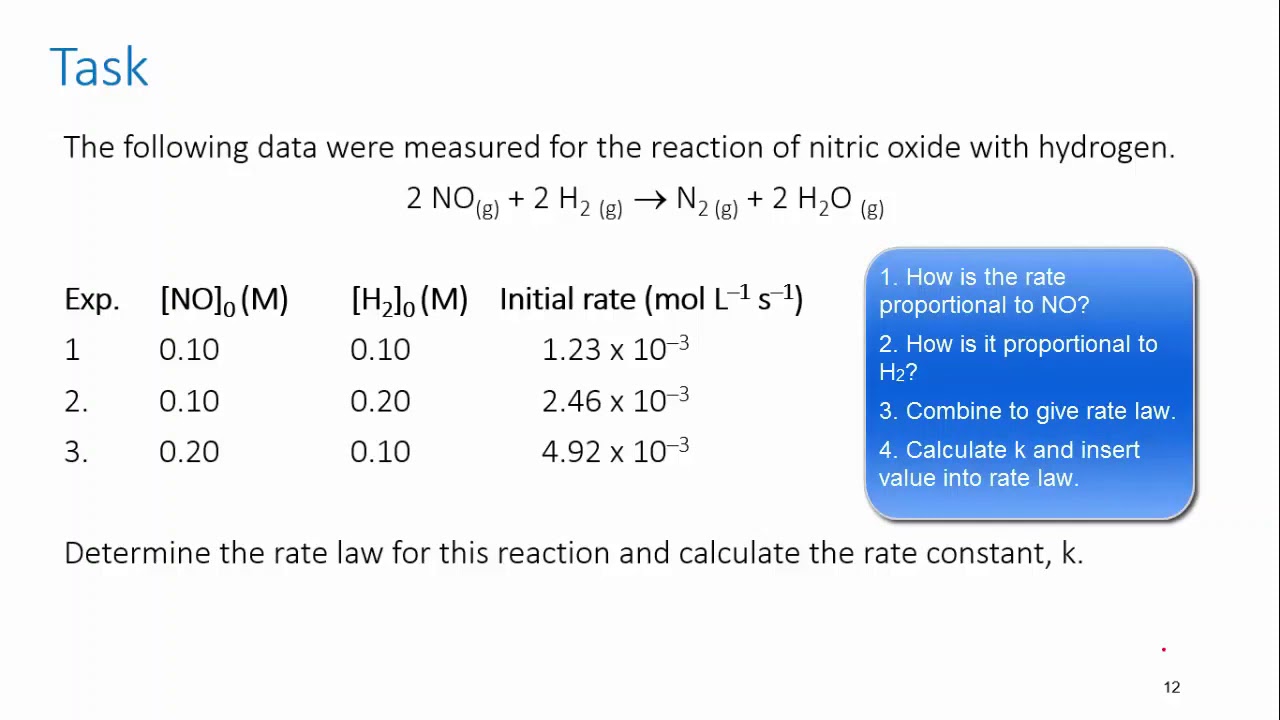
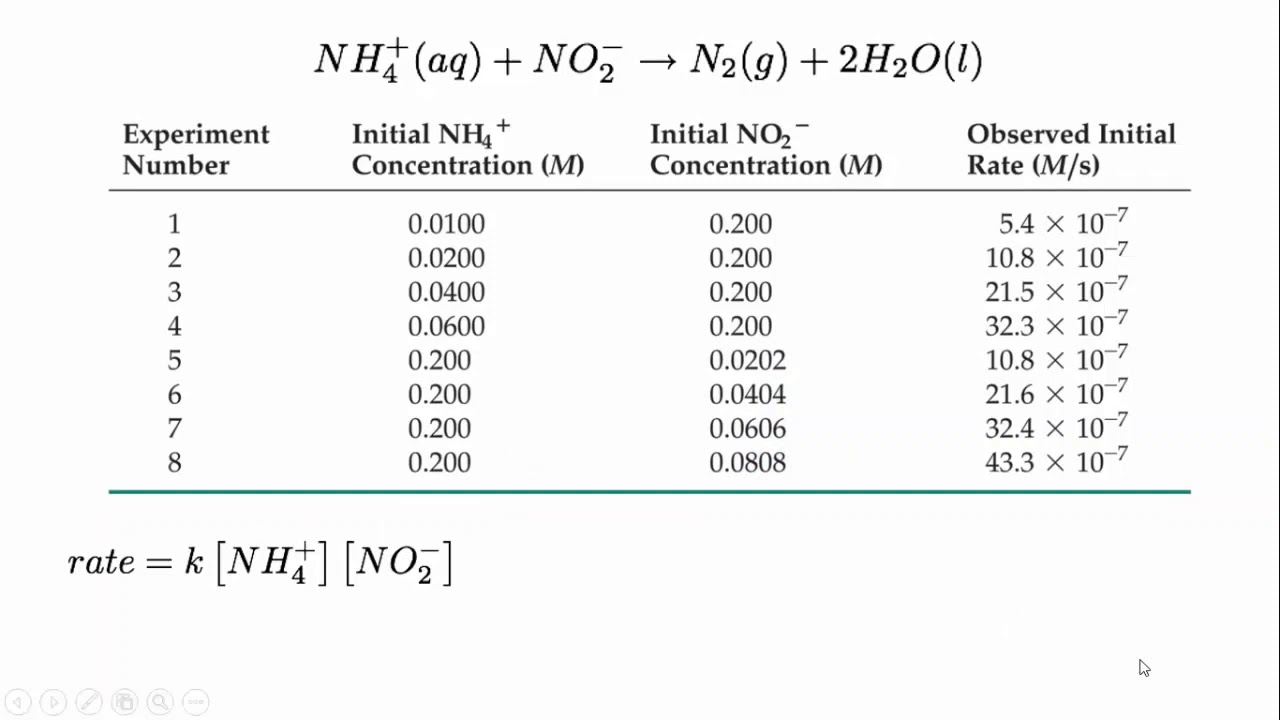
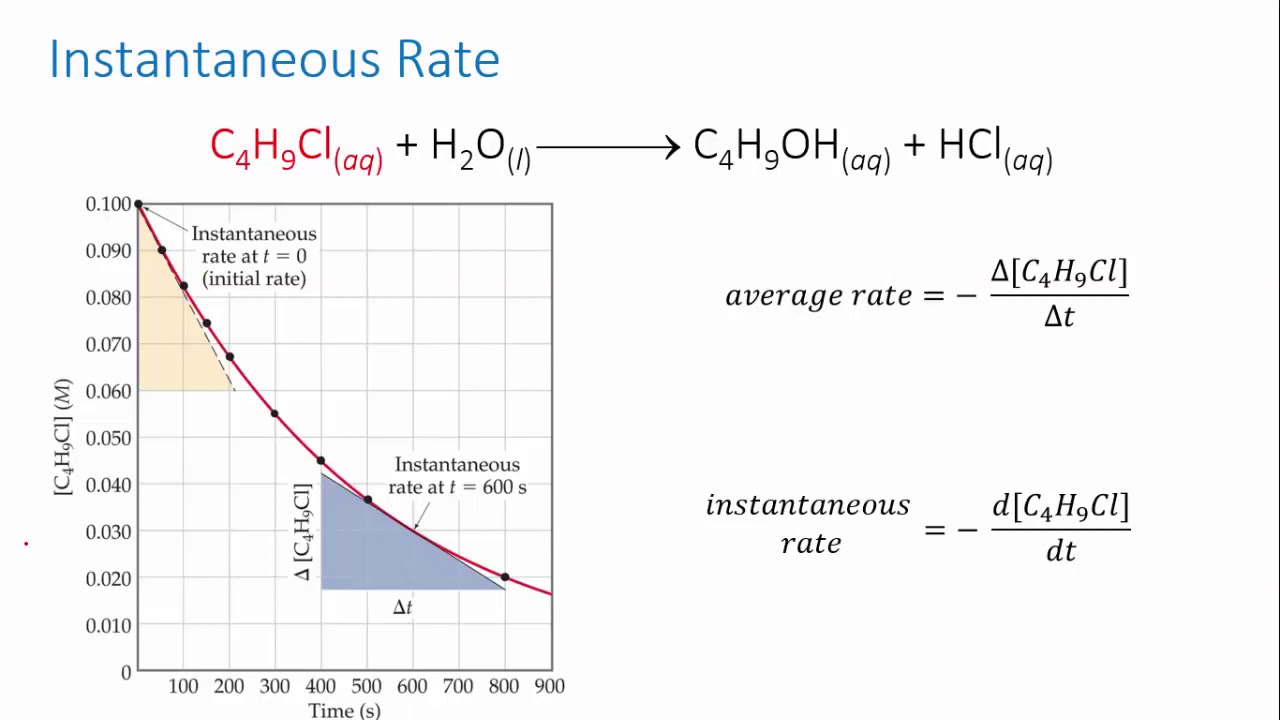
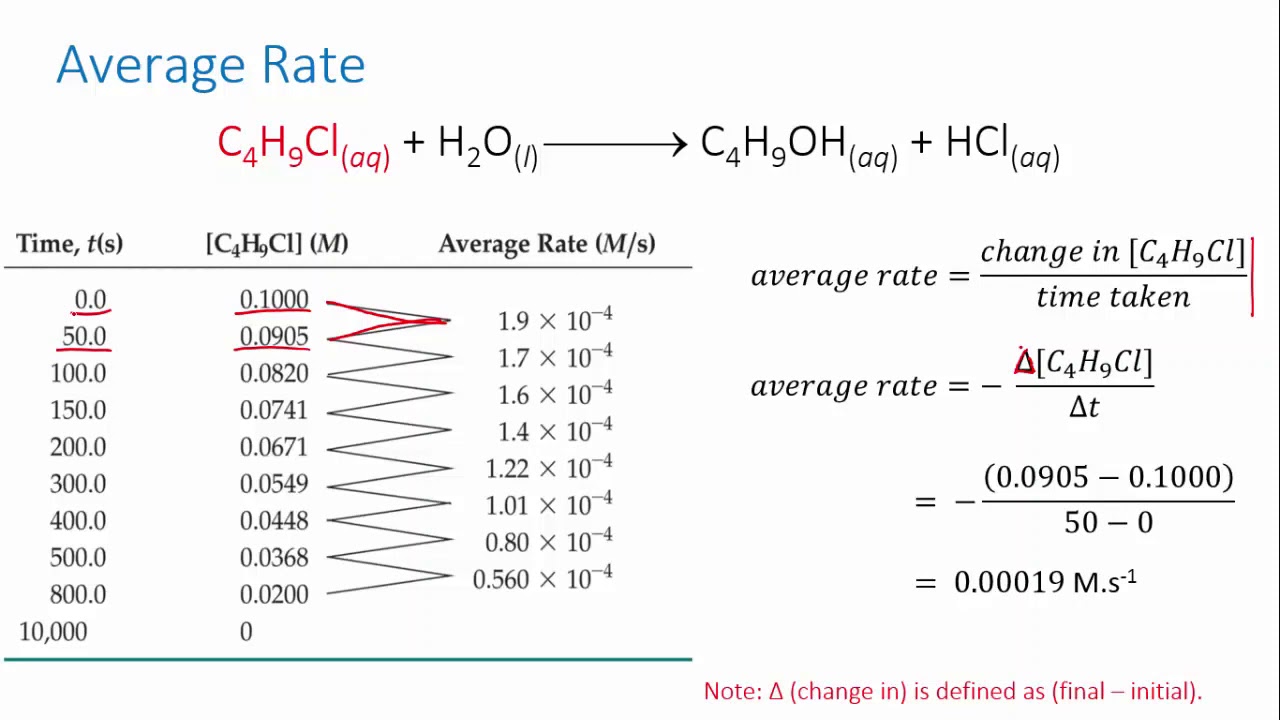
Form 4 chemistry - Reaction Rates Lesson 2 (Effects of temperature on the rate of a reaction)
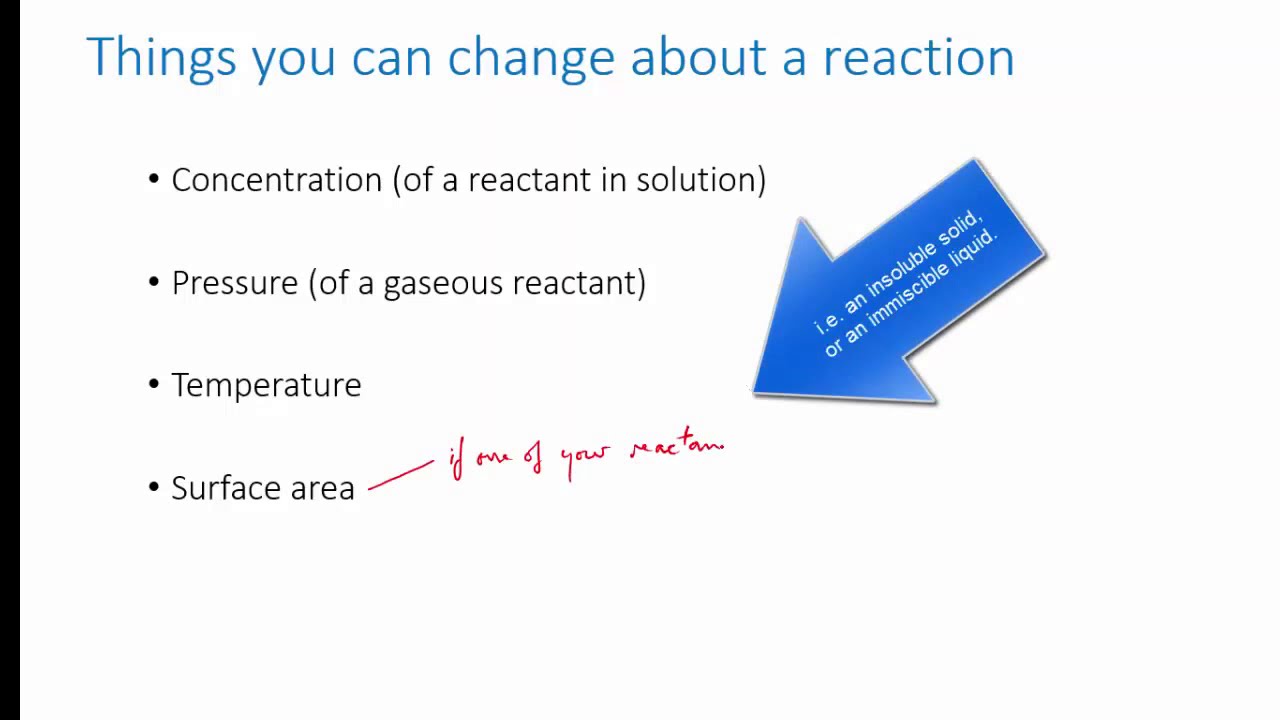
- Temperature has a significant impact on the rate of a chemical reaction.
- As the temperature increases, the rate of the reaction generally increases.
- Higher temperatures provide more energy to reactant molecules, allowing them to overcome activation energy barriers more easily and collide with greater frequency.
- This leads to more successful collisions and a faster reaction rate.
- The relationship between temperature and reaction rate is often described by the Arrhenius equation: k = Ae^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the absolute temperature.
- Temperature also influences the rate of elementary reaction steps and influences the equilibrium position of reversible reactions.
- However, extremely high temperatures can also cause unwanted side reactions or decomposition of reactants.
- It's important to note that the specific effect of temperature on the rate of a reaction depends on the nature of the reaction, the reactants, and the presence of catalysts or inhibitors.