Form 4 Chemistry - Reaction Rates Lesson 3 (Reversible reactions)
A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions, meaning that the reactants can be converted into products, and the products can also react to form the reactants again. In a reversible reaction, an equilibrium is established where the concentrations of the reactants and products remain constant over time.
Here is an example of a reversible reaction:
A + B ⇌ C + D
In the forward reaction, reactants A and B combine to form products C and D. In the reverse reaction, products C and D react to form the original reactants A and B.
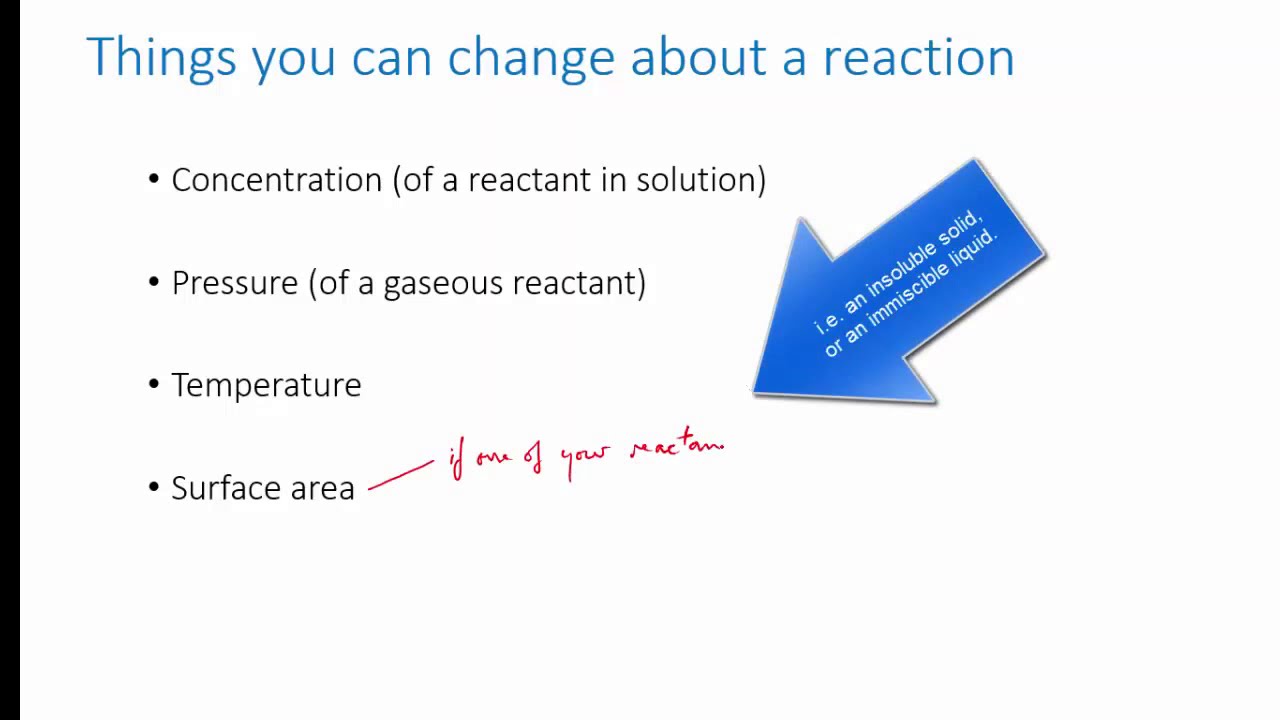
The direction of a reversible reaction is influenced by various factors such as temperature, pressure, and concentration. Le Chatelier's principle states that if a change is applied to a system at equilibrium, the equilibrium will shift to counteract the change.
For example, if the concentration of one of the reactants is increased, the equilibrium will shift towards the side with fewer moles of that reactant to reduce the excess concentration. The reaction will then favor the forward direction. Conversely, if the concentration of a product is increased, the equilibrium will shift towards the side with fewer moles of that product, favoring the reverse reaction.
Similarly, changes in pressure and temperature can also affect the equilibrium position of a reversible reaction. Increasing the pressure will favor the direction with fewer moles of gas, while decreasing the pressure will favor the direction with more moles of gas. Changes in temperature can affect the equilibrium position depending on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
Reversible reactions are essential in many chemical and biological processes, including industrial reactions, equilibrium systems, and enzymatic reactions. They provide a dynamic perspective on the behavior of chemical reactions, allowing us to understand and control them more effectively.