Form 4 chemistry - Reaction Rates Lesson 4 ( Factors that affect dynamic equilibrium)
Dynamic equilibrium is a state in which the forward and reverse reactions of a reversible reaction occur at equal rates. The position of the equilibrium can be influenced by certain factors. Here are a few factors that affect dynamic equilibrium:
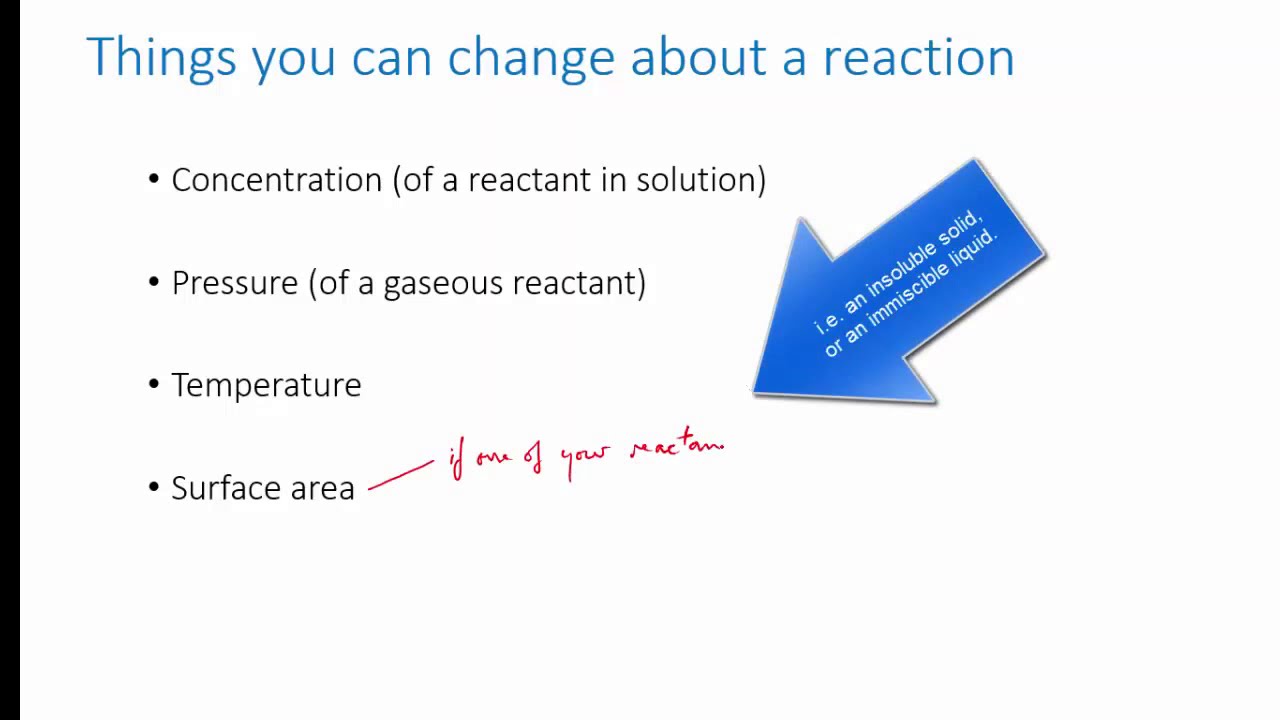
1. Changes in concentration: If the concentration of one of the reactants or products is increased, the system will try to counteract this change by shifting the equilibrium to the opposite side of the reaction, away from the added substance.
2. Changes in temperature: Changes in temperature can influence the position of the equilibrium in exothermic or endothermic reactions. Increasing the temperature will favor the endothermic reaction, and decreasing the temperature will favor the exothermic reaction.
3. Changes in pressure: Changes in pressure affect the equilibrium position in reactions with gaseous reactants and products. Increasing the pressure will decrease the volume and push the equilibrium towards the side with fewer moles, and vice versa.
4. Catalysts: Catalysts have no effect on the position of the equilibrium, but they can increase the rate at which the equilibrium is reached by lowering the activation energy of both the forward and reverse reactions.
5. Nature of reactants: The nature of the reactants involved in a reaction can affect the position of the equilibrium. If reactants are more stable than products, the equilibrium will shift towards the products side and vice versa.
6. Surface area: The surface area of the reactants can affect the rate at which the reaction occurs, thus indirectly affecting the position of the equilibrium.
In summary, the equilibrium position of a reversible reaction is affected by changes in concentration, temperature, pressure, catalysts, nature of reactants, and surface area. Understanding these factors can be useful in predicting and controlling the position of the equilibrium in various chemical reactions.