LEVEL LESSON THREE ELECTROLYSIS (Electrolytic cells)
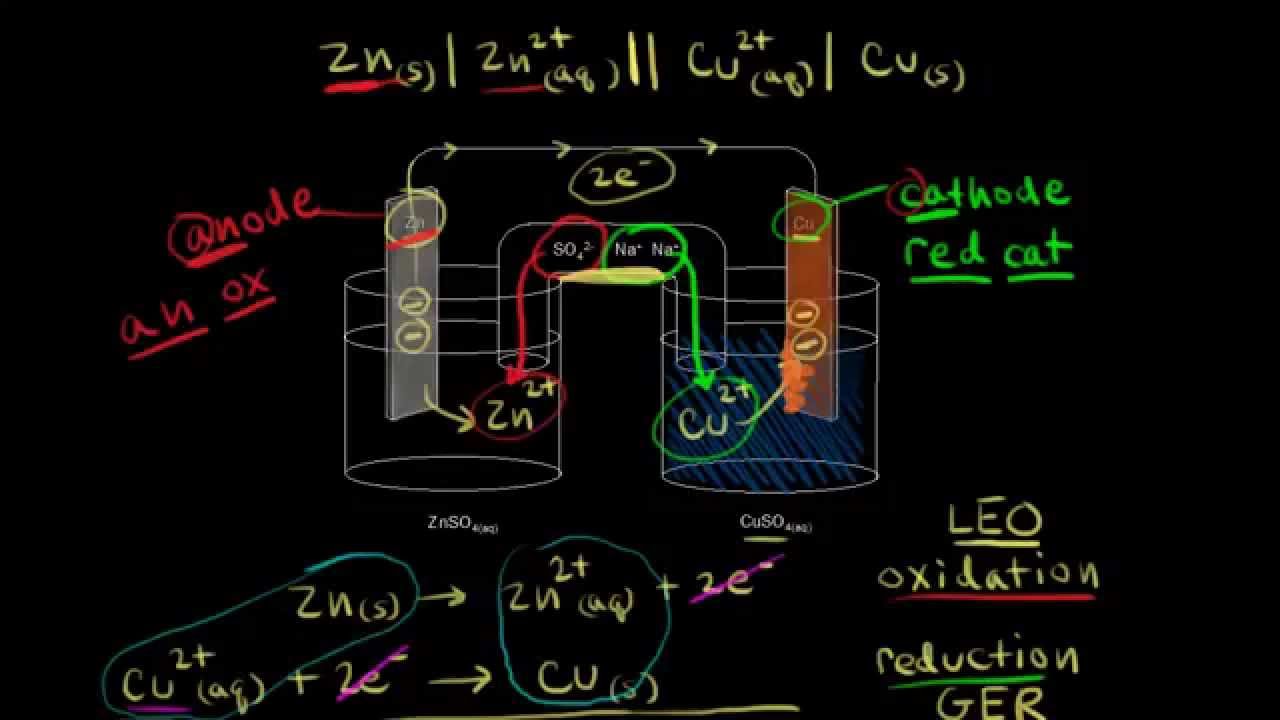
Electrolytic cells are devices that use an electric current to drive a non-spontaneous chemical reaction, known as electrolysis. They consist of an anode, a cathode, an electrolyte, and an external power source.
- Anode: The electrode connected to the positive terminal of the power source. It carries out the oxidation half-reaction by losing electrons.
- Cathode: The electrode connected to the negative terminal of the power source. It carries out the reduction half-reaction by gaining electrons.
- Electrode: Conductive surfaces where the redox reactions occur. The anode and cathode are typically made of inert materials such as platinum or graphite.
- Electrolyte: A solution or molten substance that contains ions and allows the flow of electricity. Common examples include aqueous solutions of salts or acids.
- Electric Current: The flow of charged particles (ions or electrons) through a conductor. In electrolytic cells, the power source provides the necessary electrical energy for the movement of ions.
During electrolysis, the anode attracts negatively charged ions from the electrolyte and oxidizes them. This generates electrons that flow through the external circuit to the cathode. At the cathode, positively charged ions from the electrolyte are attracted, and reduction occurs as they gain electrons.
The overall process of electrolysis involves the decomposition of the electrolyte into its constituent ions. For example, in the electrolysis of water, water molecules (H2O) are split into hydrogen ions (H+) at the cathode and oxygen gas (O2) at the anode.
Electrolytic cells have various applications, including electroplating, metal refining, electrorefining, production of chemicals, and water splitting for hydrogen production.