Reaction Rates Lesson 6 ,( industrial application of the factors that affect dynamic equilibrium)
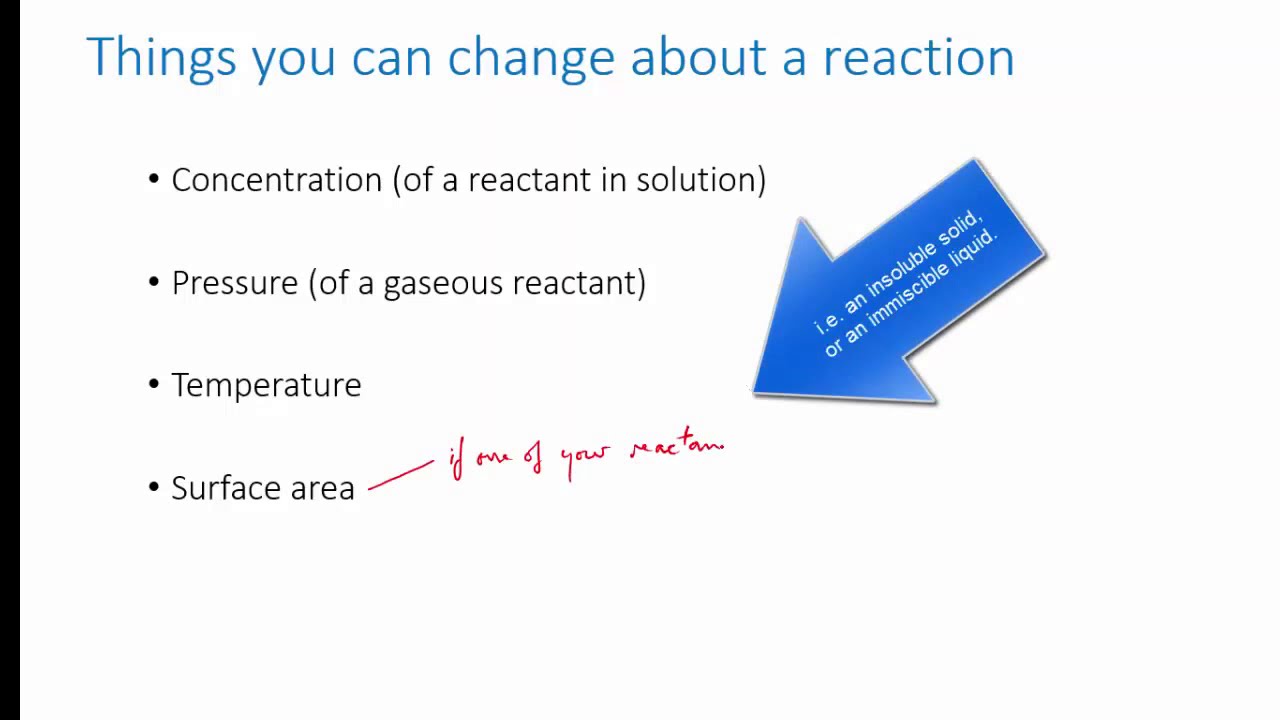
The factors affecting dynamic equilibrium such as concentration changes, temperature changes, pressure changes, catalysts, nature of reactants, and surface area have various industrial applications. Here are some examples:
1. Haber Process: The Haber Process involves the production of ammonia by combining nitrogen gas and hydrogen gas in a high-pressure, high-temperature reaction. The process takes advantage of Le Chatelier's principle, which states that increasing the pressure and decreasing the temperature favors the production of ammonia, increasing the yield of the reaction.
2. Contact Process: The Contact Process is used to produce sulfuric acid from sulfur dioxide gas, oxygen gas, and water vapor. The reaction is exothermic, and increasing the temperature will decrease the yield. However, a catalyst such as vanadium oxide is used to increase the rate of reaction, while maintaining a high yield.
3. Polymerization: In polymerization reactions, a catalyst is used to increase the rate of the reaction, and hence increase the yield of the desired product. For example, Ziegler-Natta catalysts are used in the industrial production of polyethylene, polypropylene, and other polyolefins.
4. Surface Area: In reactions involving solids, increasing the surface area of the solid will increase the rate of reaction. This principle is employed in the manufacturing of fertilizers, where the reactants are ground into fine powders to increase their surface area, resulting in a faster reaction and higher yield.
In general, the understanding and manipulation of dynamic equilibrium is an important tool for chemical industries to optimize chemical reactions and improve the efficiency of their processes.