Systems, Phases and components
Physical equilibria refer to the state of balance or stability between two or more phases of matter. Systems, phases, and components play a crucial role in understanding and analyzing physical equilibria.
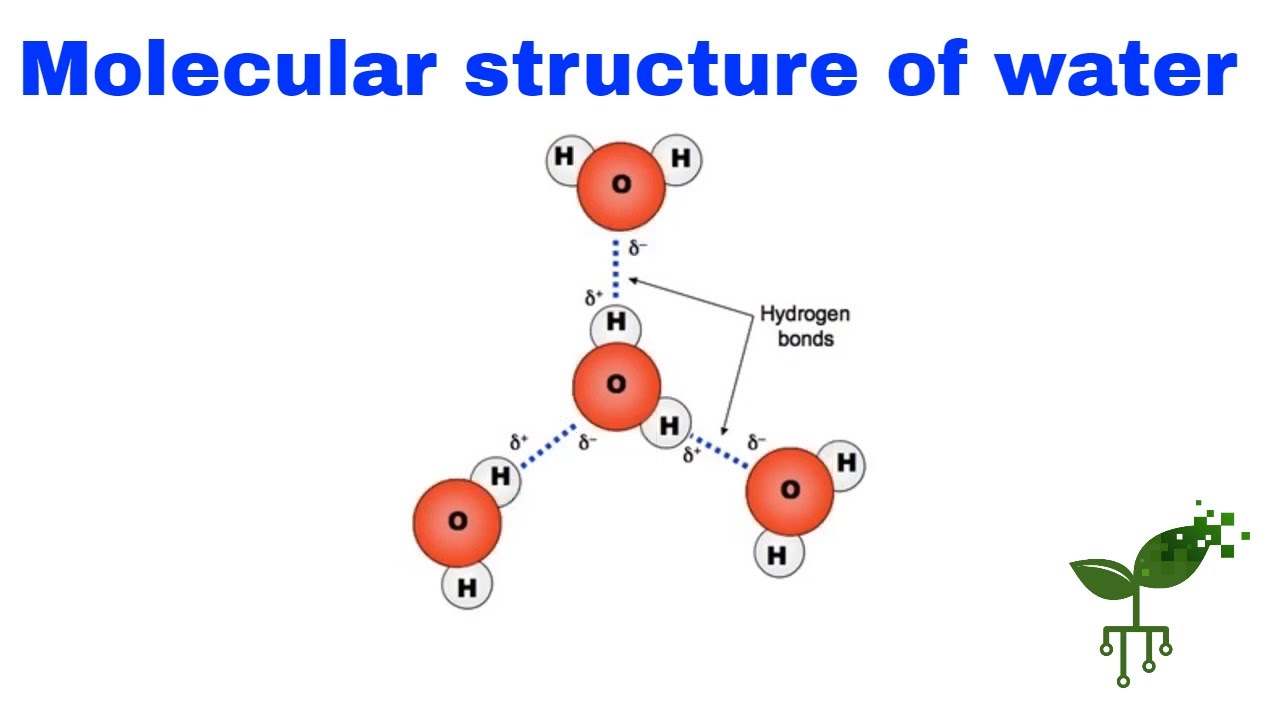
A system can refer to a collection of components that interact with each other, and physical equilibria can occur within these systems. In particular, a one-component system refers to a system containing only one substance in more than one phase. For example, a water system can be composed of water in both liquid and vapor phases. Similarly, a carbon dioxide system can consist of carbon dioxide in both liquid and gaseous phases.
Phases are the different states of matter that can coexist in a system. For example, in a water system, liquid water and water vapor can coexist in a phase equilibrium. The state of equilibrium between these two phases is referred to as the saturation point or the boiling point. Similarly, a carbon dioxide system can exhibit a phase equilibrium between carbon dioxide in a gaseous and a liquid phase.
Components refer to the individual parts that make up a larger system. In a one-component system, the component refers to the substance that exists in multiple phases. For instance, in a carbon dioxide system, the component is carbon dioxide, which can exist in both gaseous and liquid phases.
An example of physical equilibria in a one-component system is the phase transition between water in liquid and vapor states. At a particular temperature, known as the boiling point, the vapor pressure of water equals the atmospheric pressure, resulting in the formation of water vapor. Similarly, in a carbon dioxide system, at a particular temperature and pressure, the vapor pressure of carbon dioxide equals the pressure above the liquid, resulting in the formation of gaseous carbon dioxide.
In conclusion, physical equilibria in one-component systems such as water and carbon dioxide systems are critical concepts in advanced-level students' studies. Understanding the relationships between systems, phases, and components is essential for comprehending physical equilibria and their applications in various fields such as chemistry, engineering, and physics.