Chemical equilibrium and equilibrium constant Kc
this academic video for Advanced level students taking chemistry for UACE exams; it describes equilibrium constant Kc and its application with several worked examples.
In chemistry, equilibrium refers to a state where the rates of the forward and reverse reactions of a chemical reaction are equal. This means that the concentration of reactants and products remain constant over time, even though the reactions continue to occur. At equilibrium, the system is said to be in a dynamic balance.
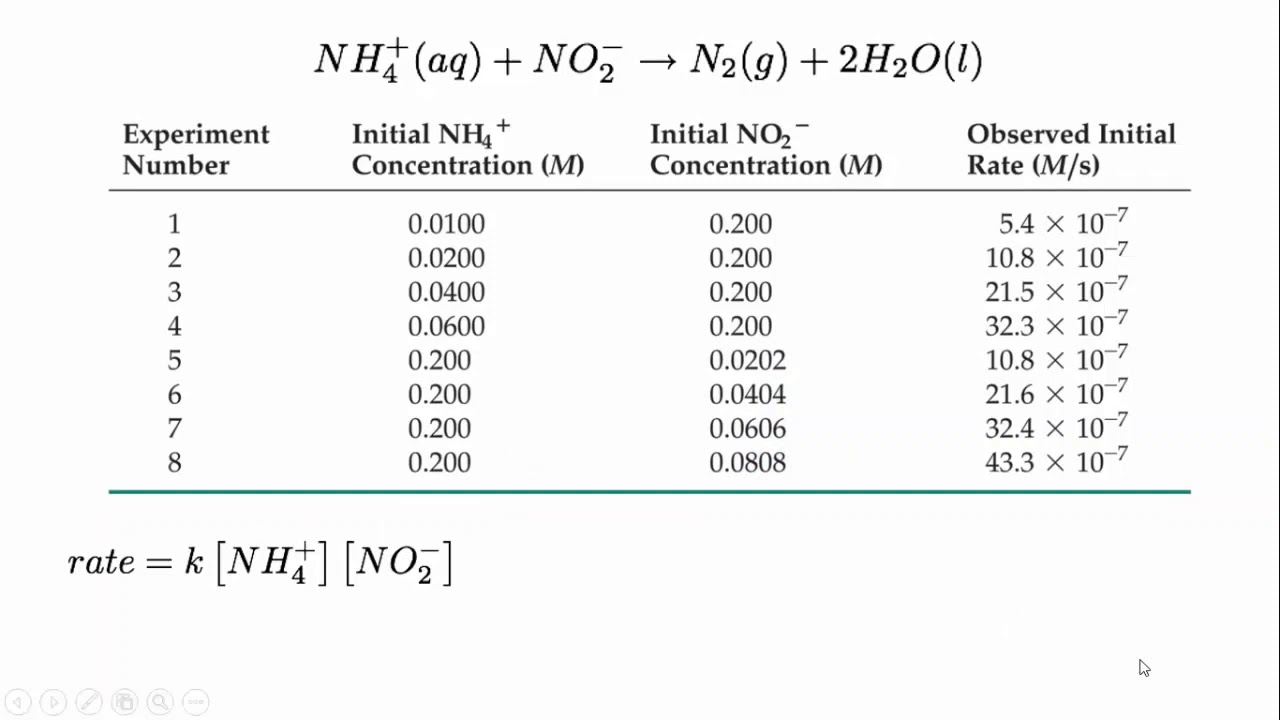
The equilibrium constant, denoted by Kc, is a numerical value that represents the ratio of the concentrations of products to the concentrations of reactants, with each concentration raised to the power of its stoichiometric coefficient in the balanced chemical equation. The equilibrium constant expression is written as:
Kc = [C]^c [D]^d / [A]^a [B]^b
Where A, B, C, and D represent the reactants and products, and a, b, c, and d represent their stoichiometric coefficients.
The equilibrium constant is a measure of the extent to which a reaction proceeds toward the formation of products. It is a constant at a given temperature and is independent of the initial concentrations of the reactants. The value of Kc reflects the relative concentrations of the reactants and products at equilibrium.
The magnitude of the equilibrium constant provides information about the position of equilibrium.
- If Kc > 1, it indicates that the equilibrium favors the products. This means that at equilibrium, the concentration of the products is higher compared to the concentrations of the reactants.
- If Kc < 1, it suggests that the equilibrium favors the reactants. At equilibrium, the concentration of the reactants is higher compared to the concentrations of the products.
- If Kc = 1, it signifies that the reactants and products are present in roughly equal concentrations at equilibrium.
The equilibrium constant provides insights into the direction in which a reaction will proceed or which species will be present in greater abundance at equilibrium. It also allows for predictions about the effect of changes in concentration, temperature, or pressure on the position of equilibrium.