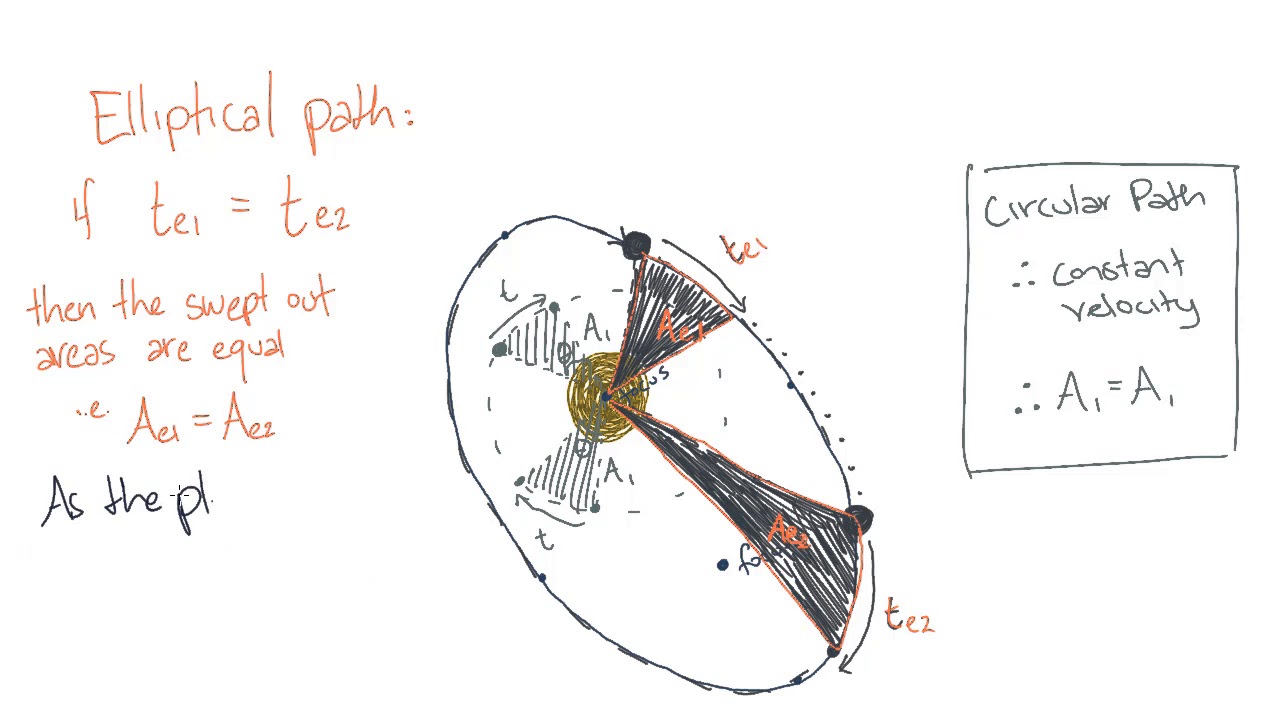
ELECTROCHEMISTRY (Faraday's laws of electrolysis)
Faraday's laws of electrolysis describe the fundamental relationship between the mass of a substance produced or consumed during electrolysis, the amount of electrical charge passed through the electrolyte, and the chemical properties of the substances involved. These laws are named after the British physicist and chemist Michael Faraday, who first described them in the early 19th century. There are two laws:
1. Faraday's First Law: The amount of a substance produced or consumed during electrolysis is directly proportional to the amount of electrical charge passed through the electrolyte.
Mathematically, this law can be expressed as:
Mass of substance produced / consumed ∝ Electrical charge passed
or,
m = zQ
where m is the mass of the substance produced or consumed, Q is the electrical charge passed, and z is a constant known as the electrochemical equivalent of the substance.
2. Faraday's Second Law: The amounts of different substances produced or consumed during electrolysis are directly proportional to their equivalent weights.
Mathematically, this law can be expressed as:
Mass of substance A / Mass of substance B = Equivalent weight of substance A / Equivalent weight of substance B
or,
m_A / m_B = EWE_A / EWE_B
where m_A and m_B are the masses of substances A and B produced or consumed, respectively, and EWE_A and EWE_B are the equivalent weights of A and B, respectively.
Faraday's laws of electrolysis are important because they explain the behavior of many electrochemical systems, and are used in a variety of applications, including electroplating, battery technology, and corrosion prevention.










![Kirchhoff's laws [IB Physics SL/HL]](https://i.ytimg.com/vi/Yrlzbq2fHRQ/maxresdefault.jpg)