Period 2 elements
chemistry of period 2 elements and diagonal relationships for Advanced Level students
The period 2 elements in the periodic table include lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne). These elements display a wide range of chemical properties and behaviors due to variations in their atomic structure and electron configurations.
1. Lithium (Li): Lithium is the lightest metal in the periodic table and is highly reactive. It readily loses its outermost electron to form a Li+ cation, making it a strong reducing agent. Lithium compounds are used in batteries, ceramics, and pharmaceuticals.
2. Beryllium (Be): Beryllium is a lightweight alkaline earth metal. It is strong, lightweight, and resistant to high temperatures, making it valuable in industries such as aerospace and nuclear power. Beryllium oxide is used as a ceramic material and a thermal conductor.
3. Boron (B): Boron is a metalloid with both nonmetallic and metallic properties. It forms covalent bonds and exhibits variations in hybridization, resulting in a diverse range of compounds. Boron compounds have numerous applications, including as fertilizers, flame retardants, and as a component in borosilicate glass.
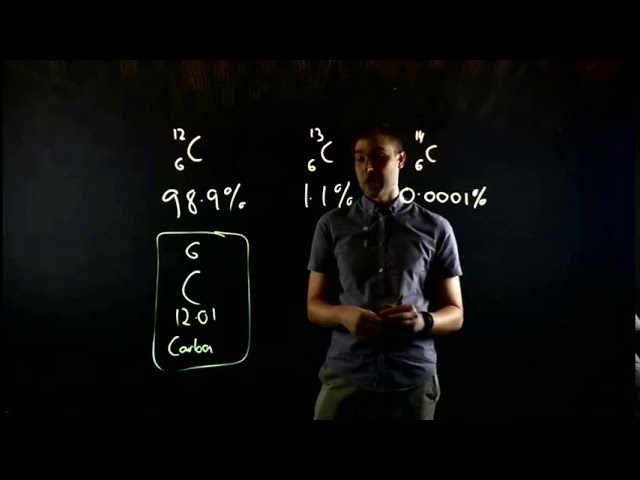
4. Carbon (C): Carbon is a nonmetal that forms the basis of organic chemistry. It has four valence electrons, allowing it to form a large variety of compounds with other elements. Carbon compounds include hydrocarbons, such as methane and ethane, as well as complex molecules like carbohydrates, proteins, and DNA.
5. Nitrogen (N): Nitrogen is a diatomic nonmetal that makes up about 78% of Earth's atmosphere. It is relatively inert and forms strong triple bonds between nitrogen atoms. Nitrogen compounds are important in fertilizers, explosives, and as a coolant in various applications.
6. Oxygen (O): Oxygen is a highly reactive nonmetal that readily forms compounds, including oxides. It is essential for respiration and combustion processes. Oxygen also plays a crucial role in the ozone layer of the Earth's atmosphere.
7. Fluorine (F): Fluorine is the most electronegative element and is highly reactive. It is a diatomic nonmetal that reacts with almost all other elements, often resulting in the release of energy. Fluorine compounds are used in toothpaste, refrigerants, and in the production of plastics.
8. Neon (Ne): Neon is a noble gas and has a completely filled valence electron shell. It is chemically inert and does not readily form compounds. Neon is commonly used in neon signs due to its bright orange-red glow when an electric current passes through it.
These period 2 elements demonstrate a wide range of chemical behaviors and applications, from highly reactive metals (such as lithium) to non-reactive noble gases (such as neon). Their properties and reactivities are a result of their electronic configurations and atomic structures.
Here are some examples of reactions involving period 2 elements:
1. Lithium and water: Lithium reacts vigorously with water to produce lithium hydroxide and hydrogen gas. The reaction is highly exothermic and liberates a large amount of heat.
2. Beryllium and oxygen: Beryllium reacts with oxygen to form beryllium oxide. This reaction is highly exothermic and releases a large amount of heat.
3. Carbon and oxygen: Carbon reacts with oxygen to form carbon dioxide. This reaction occurs during combustion processes and is responsible for the production of carbon dioxide in the atmosphere.
4. Nitrogen and hydrogen: Nitrogen reacts with hydrogen to form ammonia in the Haber process. The reaction is catalyzed by an iron catalyst and occurs at high temperature and pressure.
5. Oxygen and hydrogen: Oxygen reacts with hydrogen to form water. This reaction occurs during combustion processes, and it is also an important component of the water cycle.
6. Fluorine and metals: Fluorine reacts vigorously with metals to form metal fluorides. This reaction is highly exothermic and can result in the release of toxic fluorine gas.
7. Beryllium and acids: Beryllium reacts readily with acids to form beryllium salts and hydrogen gas. This reaction can release hydrogen gas, which may pose a hazard.
8. Boron and halogens: Boron reacts with halogens, such as chlorine and fluorine, to form boron halides. These compounds are often used as reagents in organic chemistry reactions.
9. Carbon and water: Carbon can react with water to produce carbon monoxide and hydrogen gas.
These are just a few examples of the wide range of chemical reactions that period 2 elements can participate in. The reactivity and behavior of each element are related to its electronic structure and valence electron configuration.