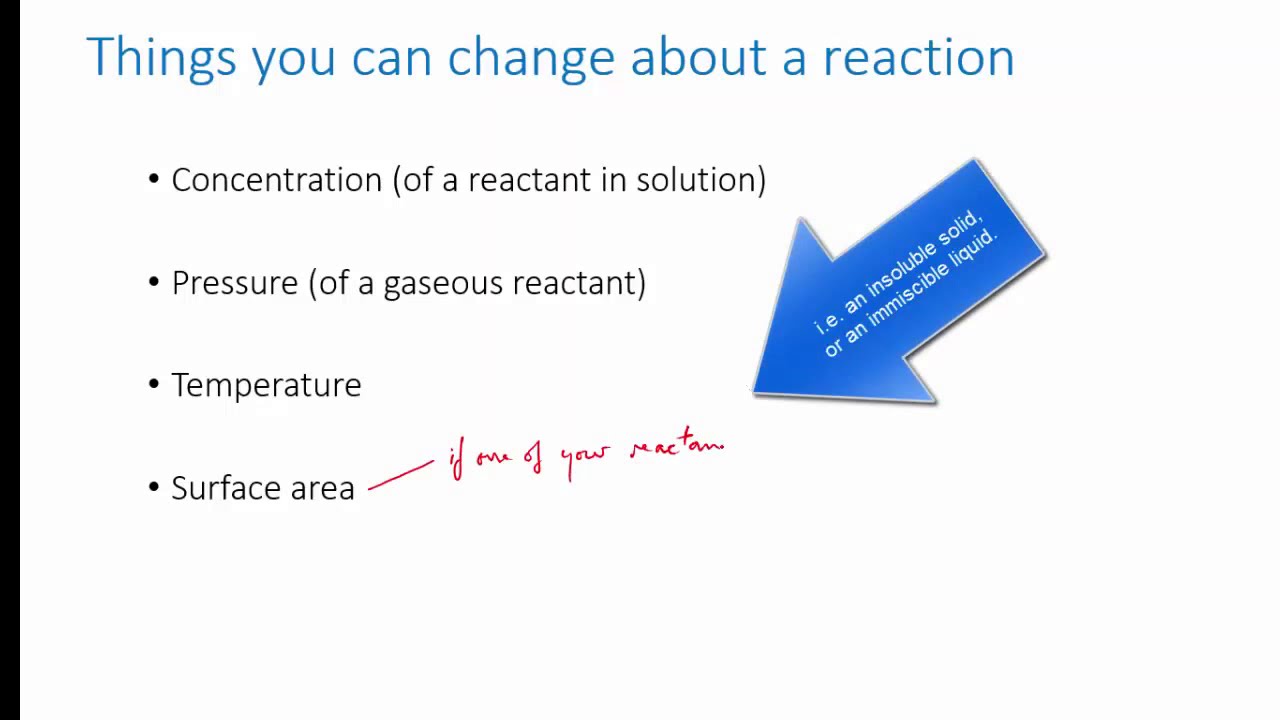
Redox reaction from dissolving zinc in copper sulfate | Chemistry | Khan Academy
What happens when you add zinc to a solution of copper sulfate? Identifying the half reactions to see what got oxidized and reduced.
Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/batter-galvanic-voltaic-cell/v/galvanic-cell-voltaic-cell?utm_source=YT&utm_medium=Desc&utm_campaign=chemistry
Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/redox-titration?utm_source=YT&utm_medium=Desc&utm_campaign=chemistry
Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful.
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #youcanlearnanything
subscribe to Khan Academy’s Chemistry channel: https://www.youtube.com/channe....l/UCyEot66LrwWFEMONv
Subscribe to Khan Academy: https://www.youtube.com/subscr....iption_center?add_us