Transition elements: Maganese
physical and chemical properties of manganese, reaction with water, air, oxidation properties of manganese VII, confirmatory tests of manganese II ions
The chemistry of manganese involves the study of the properties, reactions, and compounds of the element manganese (Mn), which belongs to the transition metal group in the periodic table. Here are some key aspects of the chemistry of manganese:
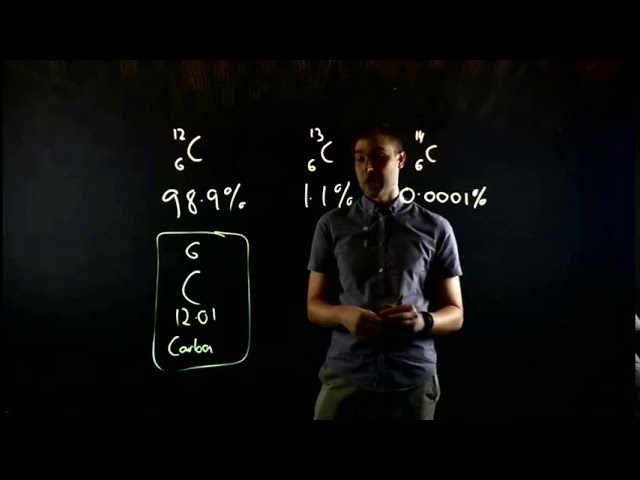
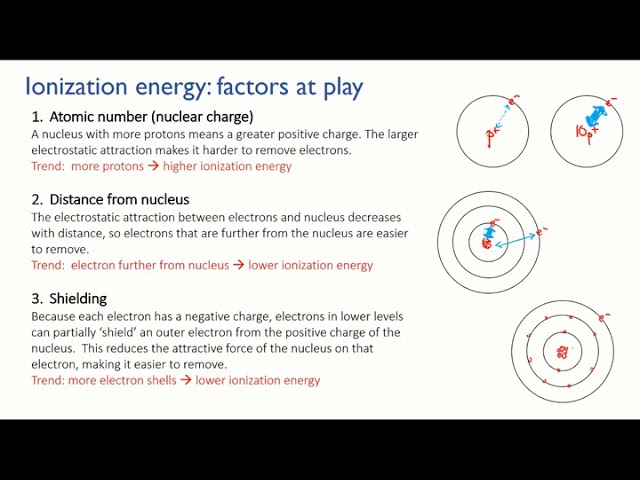
1. Oxidation states: Manganese exhibits various oxidation states ranging from -3 to +7. The most common oxidation states are +2, +4, and +7.
2. Chemical reactivity: Manganese is a moderately reactive metal. It readily reacts with halogens, sulfur, and many other non-metallic elements. It also reacts with acids to produce salts and hydrogen gas.
3. Manganese compounds: Manganese forms various compounds such as oxides (e.g., manganese dioxide), sulfides (e.g., manganese sulfide), halides (e.g., manganese chloride), and complex ions (e.g., permanganate ion).
4. Catalytic properties: Manganese, particularly in its higher oxidation states (e.g., MnO2), exhibits excellent catalytic activity. It is used as a catalyst in many chemical reactions, such as the decomposition of hydrogen peroxide.
5. Biological importance: Manganese is an essential trace element for many organisms, including plants, animals, and humans. It plays a crucial role in various biological processes, including enzyme activation, energy metabolism, and antioxidant defense.
6. Oxidation-reduction reactions: Manganese can undergo oxidation-reduction reactions by switching between different oxidation states. This ability is utilized in redox reactions and electron transfer processes.
7. Coordination chemistry: Manganese forms complex compounds with ligands in coordination chemistry. These compounds have applications in various fields, including medicine, industry, and environmental science.
8. Geological occurrence: Manganese is widely distributed in the Earth's crust. It is commonly found in ores, such as pyrolusite (MnO2) and rhodochrosite (MnCO3).
Studying the chemistry of manganese is important for understanding its properties, applications, and environmental impact. It has wide-ranging implications in fields such as materials science, environmental science, medicine, and industrial processes.