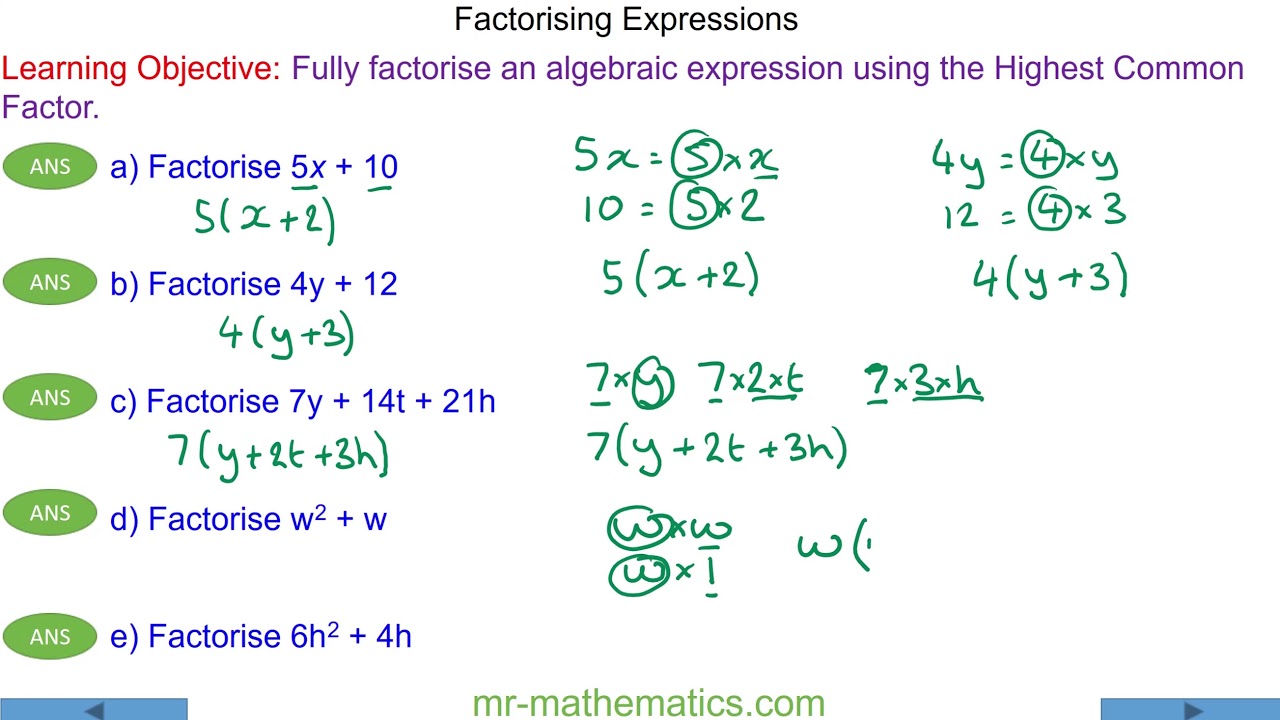
HOW TO WRITE Ksp EXPRESSIONS
A step-by-step guide on how to do so.
1. Identify the balanced chemical equation: Begin by identifying the balanced chemical equation that represents the dissolution of the compound. This equation shows how the compound dissociates into its constituent ions in aqueous solution.
2. Write the dissolution equation: Write the dissolution equation using the appropriate state symbols (s for solid and aq for aqueous). Ensure that the equation is balanced in terms of atoms and charges. For example, let's consider the dissolution of calcium carbonate (CaCO3):
CaCO3 (s) → Ca2+ (aq) + CO3^2- (aq)
3. Write the Ksp expression: The Ksp expression represents the equilibrium constant for the dissolution of the compound. It is written by taking the product of the concentrations of the constituent ions, each raised to the power of their stoichiometric coefficients. For the dissolution of calcium carbonate, the Ksp expression would be:
Ksp = [Ca2+]^1 * [CO3^2-]^1
4. Exclude solids from the expression: Only the concentrations of the dissolved ions are included in the Ksp expression. Any solid compounds are excluded. In this case, the solid calcium carbonate (CaCO3) would not be included.
5. Use brackets to denote concentration: The concentrations of the ions in the Ksp expression are typically denoted within square brackets, [ ]. However, if the ion is a polyatomic ion, such as CO3^2-, the brackets are still used, but the charge is outside the brackets.
6. Include units: It is important to include the units of concentration used in the Ksp expression. Typically, molarity (M) or moles per liter (mol/L) are used. Make sure to use consistent units for all concentrations in calculations or comparisons.
7. Don't include coefficients: The Ksp expression does not include any coefficients in front of the ions. Only the stoichiometric coefficients from the balanced chemical equation are used to determine the powers to which the concentrations are raised.
I hope this guide assists you in writing Ksp expressions. If you have any further questions or require additional explanation, please do not hesitate to reach out.
Thank you for your attention.
Best regards,