
Austins Pesarlai
|Subscribers
Liked videos
🤓😎😙
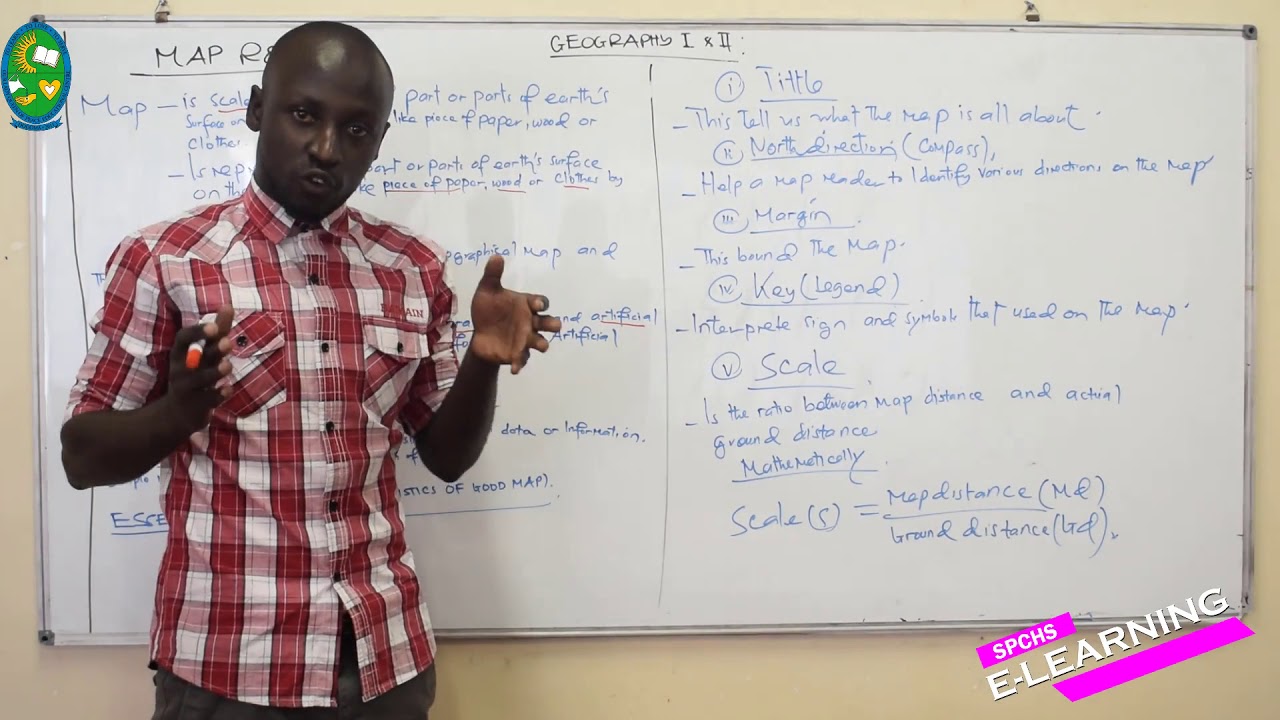
FORM II
MAIN TOPIC : MAP READING
SUB TOPIC : Introduction to map reading
Are you preparing for your IB maths exams? We've got you covered! OSC Study features exams created by IB experts in mathematics, showing you every step of every solution. Try it out for free here: https://app.oscstudy.com/
We're so excited to be able to share our exams with you!
Cheers, Mitch
Are you preparing for your IB maths exams? We've got you covered! OSC Study features exams created by IB experts in mathematics, showing you every step of every solution. Try it out for free here: https://app.oscstudy.com/
We're so excited to be able to share our exams with you!
Cheers, Mitch
Reaction rates and reversible reactions are important concepts in chemistry that are closely related.
Reaction rates refer to the speed at which a chemical reaction takes place. It measures how quickly reactants are consumed or how quickly products are formed during a reaction. Reaction rates can be influenced by various factors such as temperature, concentration, pressure, and the presence of catalysts.
On the other hand, reversible reactions are reactions that can proceed in both the forward and reverse directions. This means that reactants can form products, and products can also react to form the original reactants. Reversible reactions are denoted by a double-headed arrow (⇌) to indicate their bidirectional nature.
In reversible reactions, the forward and reverse reactions occur simultaneously, but the reaction rates may be different. The rate of the forward reaction is determined by the concentrations of the reactants, while the rate of the reverse reaction is determined by the concentrations of the products. At equilibrium, the rates of the forward and reverse reactions become equal, and the concentrations of reactants and products remain constant.
The reaction rate of a reversible reaction can be influenced by factors such as temperature, pressure, and concentration. Changing these factors can alter the position of equilibrium, which refers to the relative amounts of reactants and products in a reversible reaction system. For instance, increasing the temperature usually shifts the equilibrium towards the endothermic direction, while increasing the pressure may favor the formation of products with fewer moles.
Understanding the relationship between reaction rates and reversible reactions is important for studying chemical kinetics and thermodynamics. It allows scientists to predict and control the rates and outcomes of chemical reactions. Knowledge of reaction rates and reversible reactions is particularly useful in industrial processes, where optimizing reaction conditions can improve efficiency and yield.
- Temperature has a significant impact on the rate of a chemical reaction.
- As the temperature increases, the rate of the reaction generally increases.
- Higher temperatures provide more energy to reactant molecules, allowing them to overcome activation energy barriers more easily and collide with greater frequency.
- This leads to more successful collisions and a faster reaction rate.
- The relationship between temperature and reaction rate is often described by the Arrhenius equation: k = Ae^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the absolute temperature.
- Temperature also influences the rate of elementary reaction steps and influences the equilibrium position of reversible reactions.
- However, extremely high temperatures can also cause unwanted side reactions or decomposition of reactants.
- It's important to note that the specific effect of temperature on the rate of a reaction depends on the nature of the reaction, the reactants, and the presence of catalysts or inhibitors.
A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions, meaning that the reactants can be converted into products, and the products can also react to form the reactants again. In a reversible reaction, an equilibrium is established where the concentrations of the reactants and products remain constant over time.
Here is an example of a reversible reaction:
A + B ⇌ C + D
In the forward reaction, reactants A and B combine to form products C and D. In the reverse reaction, products C and D react to form the original reactants A and B.
The direction of a reversible reaction is influenced by various factors such as temperature, pressure, and concentration. Le Chatelier's principle states that if a change is applied to a system at equilibrium, the equilibrium will shift to counteract the change.
For example, if the concentration of one of the reactants is increased, the equilibrium will shift towards the side with fewer moles of that reactant to reduce the excess concentration. The reaction will then favor the forward direction. Conversely, if the concentration of a product is increased, the equilibrium will shift towards the side with fewer moles of that product, favoring the reverse reaction.
Similarly, changes in pressure and temperature can also affect the equilibrium position of a reversible reaction. Increasing the pressure will favor the direction with fewer moles of gas, while decreasing the pressure will favor the direction with more moles of gas. Changes in temperature can affect the equilibrium position depending on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
Reversible reactions are essential in many chemical and biological processes, including industrial reactions, equilibrium systems, and enzymatic reactions. They provide a dynamic perspective on the behavior of chemical reactions, allowing us to understand and control them more effectively.
The factors affecting dynamic equilibrium such as concentration changes, temperature changes, pressure changes, catalysts, nature of reactants, and surface area have various industrial applications. Here are some examples:
1. Haber Process: The Haber Process involves the production of ammonia by combining nitrogen gas and hydrogen gas in a high-pressure, high-temperature reaction. The process takes advantage of Le Chatelier's principle, which states that increasing the pressure and decreasing the temperature favors the production of ammonia, increasing the yield of the reaction.
2. Contact Process: The Contact Process is used to produce sulfuric acid from sulfur dioxide gas, oxygen gas, and water vapor. The reaction is exothermic, and increasing the temperature will decrease the yield. However, a catalyst such as vanadium oxide is used to increase the rate of reaction, while maintaining a high yield.
3. Polymerization: In polymerization reactions, a catalyst is used to increase the rate of the reaction, and hence increase the yield of the desired product. For example, Ziegler-Natta catalysts are used in the industrial production of polyethylene, polypropylene, and other polyolefins.
4. Surface Area: In reactions involving solids, increasing the surface area of the solid will increase the rate of reaction. This principle is employed in the manufacturing of fertilizers, where the reactants are ground into fine powders to increase their surface area, resulting in a faster reaction and higher yield.
In general, the understanding and manipulation of dynamic equilibrium is an important tool for chemical industries to optimize chemical reactions and improve the efficiency of their processes.
Dynamic equilibrium is a state in which the forward and reverse reactions of a reversible reaction occur at equal rates. The position of the equilibrium can be influenced by certain factors. Here are a few factors that affect dynamic equilibrium:
1. Changes in concentration: If the concentration of one of the reactants or products is increased, the system will try to counteract this change by shifting the equilibrium to the opposite side of the reaction, away from the added substance.
2. Changes in temperature: Changes in temperature can influence the position of the equilibrium in exothermic or endothermic reactions. Increasing the temperature will favor the endothermic reaction, and decreasing the temperature will favor the exothermic reaction.
3. Changes in pressure: Changes in pressure affect the equilibrium position in reactions with gaseous reactants and products. Increasing the pressure will decrease the volume and push the equilibrium towards the side with fewer moles, and vice versa.
4. Catalysts: Catalysts have no effect on the position of the equilibrium, but they can increase the rate at which the equilibrium is reached by lowering the activation energy of both the forward and reverse reactions.
5. Nature of reactants: The nature of the reactants involved in a reaction can affect the position of the equilibrium. If reactants are more stable than products, the equilibrium will shift towards the products side and vice versa.
6. Surface area: The surface area of the reactants can affect the rate at which the reaction occurs, thus indirectly affecting the position of the equilibrium.
In summary, the equilibrium position of a reversible reaction is affected by changes in concentration, temperature, pressure, catalysts, nature of reactants, and surface area. Understanding these factors can be useful in predicting and controlling the position of the equilibrium in various chemical reactions.
A step-by-step guide on how to do so.
1. Identify the balanced chemical equation: Begin by identifying the balanced chemical equation that represents the dissolution of the compound. This equation shows how the compound dissociates into its constituent ions in aqueous solution.
2. Write the dissolution equation: Write the dissolution equation using the appropriate state symbols (s for solid and aq for aqueous). Ensure that the equation is balanced in terms of atoms and charges. For example, let's consider the dissolution of calcium carbonate (CaCO3):
CaCO3 (s) → Ca2+ (aq) + CO3^2- (aq)
3. Write the Ksp expression: The Ksp expression represents the equilibrium constant for the dissolution of the compound. It is written by taking the product of the concentrations of the constituent ions, each raised to the power of their stoichiometric coefficients. For the dissolution of calcium carbonate, the Ksp expression would be:
Ksp = [Ca2+]^1 * [CO3^2-]^1
4. Exclude solids from the expression: Only the concentrations of the dissolved ions are included in the Ksp expression. Any solid compounds are excluded. In this case, the solid calcium carbonate (CaCO3) would not be included.
5. Use brackets to denote concentration: The concentrations of the ions in the Ksp expression are typically denoted within square brackets, [ ]. However, if the ion is a polyatomic ion, such as CO3^2-, the brackets are still used, but the charge is outside the brackets.
6. Include units: It is important to include the units of concentration used in the Ksp expression. Typically, molarity (M) or moles per liter (mol/L) are used. Make sure to use consistent units for all concentrations in calculations or comparisons.
7. Don't include coefficients: The Ksp expression does not include any coefficients in front of the ions. Only the stoichiometric coefficients from the balanced chemical equation are used to determine the powers to which the concentrations are raised.
I hope this guide assists you in writing Ksp expressions. If you have any further questions or require additional explanation, please do not hesitate to reach out.
Thank you for your attention.
Best regards,
In this video, I have tried to answer all Questions of UNEB Sub ICT 2023 Paper 3. Hopefully, it helps you in your revision and practice, and those who didn't do the paper can learn more from it as well.
I welcome comments on guidance in case a certain Roman (Question) was not well addressed.
Subscribe and Share
Thank You
In this video get to know the Top 60 Best Microsoft PowerPoint Tips, Tricks, Secrets and Hacks
Here is the link http://tinyurl.com/bdcuzkkc to access the presentation template that was used during the session for you to fill in as you practice the tips and then link to the amazing templates https://www.yaaka.cc/product/d....ownload-powerpoint-s
Here are the Top 60 Best Microsoft PowerPoint Tips, Tricks, Secrets, and Hacks you need to know;
1. Slide Size
2. Slide Layout
3. Using Ribbon Shortcuts (alt – key)
4. Stock images, (insert tab) and
5. 3-D models,
6. icons,
7. online pictures
8. stock videos
9. Remove background e.g. from stock images
10. Shape Edit points, customize it a more eg rounded rectangle, partial circle
11. Gridlines and Guides
12. Inserting maps
13. Inserting graphs📊
14. Chart animations – eg float in, animation pane, by series effect, combo charts
15. Quick alignment of shapes, middle, left, etc., and either vertical or horizontally
16. Grouping objects, front and back
17. Add Quick Access Toolbar (QAT)
18. Alt + shift+ order (up arrow/down key) to arrange a list
19. Animations to images, custom path/motion path
20. Photo Album from Insert
21. Reuse slides
22. Adding speaker notes/presenter notes
23. Creating animated gifs (export) have animations and transitions then SmartArt’s
24. Embed fonts. – file-options-save-check-embed fonts in the file (when you need to share it with your other person for presenting)
25. Media compression tool – file-info-compress media
26. Shape intersects, Venn diagrams (fragment) (merge shapes)
27. Picture fill (shape fill)
28. Morph and enhanced morph (https://youtu.be/s7jbBLJZ6a0 ) draw two circles and duplicate
29. Eye dropper for shape fill and outline
30. Copy objects with ctrl
31. Copy objects with ctrl + Shift
32. Duplicate objects with ctrl + Shift then F4 key Twice
33. Presentation mode shortcut key
a. Press F1 while in slide show
34. Advanced crop options
35. Hold shift as you draw shapes for perfect geometric shapes
36. Convert Text put in a text box into smart art – home, paragraph the smart art (for example instead of bullets)
37. Default Textbox in a presentation (draw format it then right click set as default textbox)
38. Filler text Check in speaker notes area (=lorem()
39. Zoom Slide Feature - \ insert zoom
40. Change colors at Once
41. Importing word file into ppt
42. Group and Align, Shape and Text
43. Drawing tool – then ink to shape, text, math
44. Lock drawing mode (for connecting organizational charts)
45. Text With Image – intersect UNDER MERGE SHAPES
46. Grid Image (draw similar squares/circles, group them, then fill them with an image)
47. Save as JPEG / PNG
48. Symbol as bullet points
49. Adding media ie video, audio/music
50. Printing options ie pdf or handouts
51. screenshot
52. Record
53. Designer,
54. dictate
55. Slide Show loop
56. linking slides and Hyperlinks
57. slide sorter view
58. auto correct
59. blank screen
60. recover files (click file open, recover unsaved files)
61. tell me what to do / Search
62. Export presentation to video
Here is the link http://tinyurl.com/bdcuzkkc to access the presentation template that was used during the session for you to fill in as you practice the tips http://tinyurl.com/bdcuzkkc
Here is also a link to download amazing templates for your presentation https://www.yaaka.cc/product/d....ownload-powerpoint-s
call +256709716945 or +256770730170 for assistance in getting these templates
Link to the entire session https://youtu.be/SECY2LdvwqM #ultimatemultimediaconsult for other professional skills development sessions
#microsoft #powerpoint
Properties of alkeness
sources ofalkenes
This video was a live show on UBC-Star Tv in which students were introduced to the dos and donts of organic synthesis
Classification of alkylhalides (primary, secondary and tertiary alkyl halides with example.preparations of alkylhalides from alkanes, alkenes, and alcohols
## Classification of Alkyl Halides:
Alkyl halides, also known as haloalkanes, are organic compounds containing a halogen atom (F, Cl, Br, I) bonded to a saturated carbon atom. They are classified based on the number of carbon atoms attached to the carbon bearing the halogen atom:
* **Primary (1°):** One carbon atom is attached to the carbon with the halogen. (e.g., CH₃Cl - Chloromethane)
* **Secondary (2°):** Two carbon atoms are attached to the carbon with the halogen. (e.g., CH₃CH₂Cl - Chloroethane)
* **Tertiary (3°):** Three carbon atoms are attached to the carbon with the halogen. (e.g., (CH₃)₃CCl - Trichloromethane)
## Preparations of Alkyl Halides:
**1. From Alkanes:**
* **Free radical halogenation:** Reaction of alkanes with halogen molecules (Cl₂, Br₂) in the presence of light or heat. This is a non-selective method and can lead to a mixture of products. (e.g., Methane + Cl₂ -> Chloromethane, Dichloromethane, etc.)
**2. From Alkenes:**
* **Electrophilic addition of hydrogen halides (HX):** Addition of HX (HCl, HBr, HI) across the double bond of an alkene. Follows Markovnikov's rule, where the halogen atom gets attached to the more substituted carbon. (e.g., Propene + HCl -> 2-Chloropropane)
**3. From Alcohols:**
* **Reaction with hydrogen halides (HX):** Conversion of alcohols to alkyl halides by replacing the hydroxyl group (-OH) with a halogen atom. Requires a catalyst for primary and secondary alcohols, not for tertiary alcohols. (e.g., Ethanol + HCl -> Chloroethane + H₂O)
* **Reaction with thionyl chloride (SOCl₂):** Effective method for converting primary and secondary alcohols to alkyl halides. (e.g., Ethanol + SOCl₂ -> Chloroethane + SO₂ + HCl)
**Note:** The specific reaction conditions and choice of method depend on the desired alkyl halide and the starting material.
Modes of conduction of substances, common terms used in electrolysis
## Modes of Conduction in Substances:
There are three main modes of conduction observed in different substances:
**1. Metallic Conduction:**
* **Description:** Involves the movement of **free electrons** within a metallic lattice. These electrons are not bound to any specific atom and can move freely throughout the metal.
* **Examples:** Metals like copper, aluminum, and silver are good conductors of electricity due to the presence of a large number of free electrons.
**2. Ionic Conduction:**
* **Description:** Occurs in **electrolytes** (molten salts or ionic solutions) where **ions** move through the solution carrying the charge.
* **Examples:** Aqueous solutions of salts like NaCl or molten salts like NaCl (liquid) conduct electricity through the movement of Na⁺ and Cl⁻ ions.
**3. Electronic Conduction in Semiconductors:**
* **Description:** Involves the movement of both **electrons** and **holes** (the absence of an electron in the valence band) in semiconductors. The conductivity can be controlled by applying external factors like doping or electric fields.
* **Examples:** Materials like silicon and germanium exhibit semiconducting behavior, where their conductivity can be tailored for various applications in electronics.
## Common Terms Used in Electrolysis:
* **Electrolyte:** A substance that conducts electricity due to the presence of free ions.
* **Electrode:** An electrical conductor that is in contact with an electrolyte.
* **Anode:** The positive electrode where oxidation occurs.
* **Cathode:** The negative electrode where reduction occurs.
* **Electrolysis:** The process of using electrical energy to drive a non-spontaneous chemical reaction.
* **Electrolysis products:** The substances formed at the electrodes during electrolysis.
* **Electrolytic cell:** A device used to carry out electrolysis, consisting of electrodes, an electrolyte, and a power source.
* **Aqueous electrolysis:** Electrolysis involving water as the electrolyte.
* **Electroplating:** The deposition of a metal onto the cathode from a metal-containing solution.
* **Electrorefining:** The purification of a metal by removing impurities that go into the solution during electrolysis.
Understanding these terms and the different modes of conduction is crucial for comprehending the principles and applications of electrolysis in various fields like electroplating, battery technology, and chemical production.
Common terms used in electrolysis and explanation of the changes that take place during electrolysis
Common Terms Used in Electrolysis:
Electrolyte: A substance that conducts electricity due to the presence of free ions. These ions can be dissolved in a solvent (like aqueous solutions) or molten.
Electrode: An electrical conductor in contact with an electrolyte. There are two types:
Anode: The positive electrode where oxidation occurs. Electrons flow out of the anode.
Cathode: The negative electrode where reduction occurs. Electrons flow into the cathode.
Electrolysis: The process of using electrical energy to drive a non-spontaneous chemical reaction. An external power source provides the energy to overcome the activation energy barrier of the reaction.
Electrolysis products: The substances formed at the electrodes during electrolysis. These products depend on the specific reaction occurring.
Electrolytic cell: A device used to carry out electrolysis, consisting of electrodes, an electrolyte, and a power source.
Changes During Electrolysis:
Electrolysis involves several key changes:
Electrical energy to chemical energy: The external power source provides electrical energy, which is converted into chemical energy to drive the non-spontaneous reaction.
Oxidation at the anode: Anions from the electrolyte lose electrons at the anode, undergoing oxidation. This can involve the electrode itself being oxidized or the oxidation of ions in the electrolyte.
Reduction at the cathode: Cations from the electrolyte gain electrons at the cathode, undergoing reduction.
Movement of ions: Ions in the electrolyte migrate towards the oppositely charged electrode to maintain electrical neutrality.
Formation of electrolysis products: The products of the oxidation and reduction reactions at the electrodes form the final electrolysis products.
Example: Electrolysis of water (H₂O):
Electrolyte: Aqueous solution of sodium chloride (NaCl)
Anode: 2Cl⁻ → Cl₂ + 2e⁻ (Chlorine gas is produced at the anode)
Cathode: 2H₂O + 2e⁻ → H₂ + 2OH⁻ (Hydrogen gas is produced at the cathode)
Overall reaction: 2H₂O → 2H₂ + O₂ (decomposition of water)
Note: This is a simplified example. The specific reactions and products depend on the nature of the electrolyte and the applied voltage.
This is a zoom recorded video that will introduce A level students to THERMOCHEMISTRY. It was created to enhance learning during the Covid-19 Lockdown. Please like and share and subscribe to our you tube channel for more such videos.
How Moya David surprised Mountain View School Grade 6 & Class 8 candidates ahead of their exams.
Wish our candidates success doing KEPSEA (Grade 6) and KCPE (Class 8) success in the comment section.
Video produced by Coolads Media Limited.
All Rights Reserved!
😍


![Flux and Faraday’s law [IB Physics HL]](https://i.ytimg.com/vi/qWs7fuEMHzs/maxresdefault.jpg)
![Lenz’s law [IB Physics HL]](https://i.ytimg.com/vi/lVgEMVNVnGI/maxresdefault.jpg)
















